Thoracic Aortic, Abdominal Aortic, and Lower Extremity Interventions
Beau M. Hawkins MD
Douglas E. Drachman MD, FACC, FSCAI
Interventional cardiovascular specialists encounter peripheral artery disease (PAD) in all aspects of routine clinical practice. Even the most skilled operators will occasionally cause peripheral vascular complications that require interventional therapy. As such, an understanding of the basic principles and indications for peripheral revascularization is critical for all interventionalists, including those who do not routinely perform noncoronary interventions. This chapter succinctly summarizes thoracic, aortic, and lower extremity interventions with an emphasis on what should be anticipated on the interventional cardiology board examination.
THORACIC AORTA
Subclavian and Brachiocephalic Intervention
Obstructive disease of the major aortic arch vessels supplying the upper extremities is a common condition affecting up to 7% of individuals in select populations (1). When atherosclerotic in nature, subclavian and brachiocephalic disease is predominantly ostial or proximal in location; but other conditions such as fibromuscular dysplasia, medium- and large-vessel vasculitides (e.g., Takayasu arteritis, giant cell arteritis), thoracic outlet syndrome, or radiation-induced disease may cause lesions in more distal locations (2).
Symptoms of subclavian obstruction include arm claudication manifest by fatigue, paresthesia, or pain during exertion. Proximal left subclavian stenosis may also impede antegrade flow through the left vertebral or internal mammary (LIMA) arteries, resulting in symptoms of vertebrobasilar insufficiency or angina when the LIMA has been used for coronary artery bypass grafting (CABG).
Revascularization of the brachiocephalic and subclavian arteries is indicated in the presence of significant symptoms such as claudication, vertebrobasilar insufficiency, or angina. Additionally, when the LIMA is required as a conduit for CABG surgery, empiric revascularization of left subclavian stenosis is appropriate, even in the absence of symptoms (2). Importantly, the identification of flow reversal in the vertebral artery—a commonly encountered finding on Doppler ultrasound examinations—should not prompt revascularization in asymptomatic patients unless the internal mammary is needed for bypass purposes.
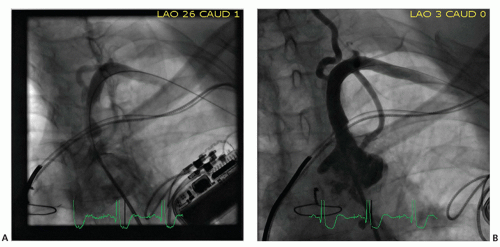
Technically, percutaneous revascularization of the subclavian and brachiocephalic arteries is successful in >95% of cases (3). No randomized data exist comparing open surgical revascularization with stenting. The femoral approach is most often utilized, although brachial access may facilitate treatment of chronic total occlusions, where it may be difficult to localize the vessel’s origin from the aortic arch or to maintain adequate catheter support to cross the occlusion. The successful use of radial access has been reported (4), but is less commonly utilized because systems larger than 6F are frequently required to accommodate larger stent platforms. Ostial and proximal lesions are generally treated with balloon-expandable stents as radial force is desirable and this region is not exposed to extrinsic compression. For lesions located in the more distal portions of these vessels, self-expanding stents may be preferred to accommodate for the increased mobility of the vessels in these regions associated with upper extremity movement. If, however, the lesion is just distal to the origin of the mammary or vertebral arteries, brachial or radial access should be considered to ensure that self-expanding stent deployment does not inadvertently cover the origins of these important vessels. Atheroembolization, while uncommon, represents a devastating potential complication, which occurs because of the direct route to the cerebral circulation through the vertebral artery. Some operators advocate the use of cerebral embolic protection at the time of treatment of bulky or angiographically “worrisome” subclavian or brachiocephalic lesions (5), but no convincing data are available to validate this strategy. Figure 35-1 shows recanalization after stenting of left subclavian artery.
Coarctation
Aortic coarctation is a discrete narrowing of the thoracic aorta that preferentially occurs in proximity to the ligamentum arteriosum. It is the sixth most common form of congenital heart disease, and commonly occurs with other congenital lesions, classically in association with bicuspid aortic valve. Coarctation in children and adults is most commonly manifested as hypertension, and should be suspected in the presence of a brachial-femoral artery pulse delay (6). Individuals with unrepaired coarctation are at an increased risk of cardiovascular events, including stroke, heart failure, and death.
Endovascular or surgical repair is recommended in individuals with a gradient of >20 mm Hg across the segment of coarctation. Since collateralization may reduce the detectable gradient across the coarcted segment, intervention should also be considered in lower gradient states if diagnostic imaging demonstrates significant collateral flow. Surgical repair and endovascular angioplasty are both acceptable modes of therapy. In general, surgical repair is reserved for those with compelling anatomical indications such as branch artery involvement or associated large aneurysmal dilatation. Angioplasty is associated with significant recurrence rates; primary stenting is generally preferred. The risk of aneurysm formation following surgical or endovascular treatment of coarctation is significant, and patients with a history of coarctation repair should receive annual follow-up with thoracic aortic imaging at regular intervals (7).
ABDOMINAL AORTA
Abdominal aortic aneurysm (AAA) remains a prevalent condition that poses a significant risk of death from rupture. Tobacco use, Caucasian race, and male gender are the classic associated risk factors, while, interestingly, diabetes appears protective (8). Current recommendations support one-time ultrasound screening for AAA in males aged 65 to 74 with a history of smoking (9).
Open surgical repair has historically been considered the definitive therapy, though in recent times, endovascular repair (EVAR) has been increasingly utilized owing to its lower periprocedural risk (10). Repair should be offered for aneurysms that are >5.5 cm, or for those of any size that are symptomatic. Regarding the choice of repair, there remains considerable debate. Current ACC/AHA guidelines support open surgical repair for acceptable operative candidates. EVAR is a reasonable option for those at high risk for open repair with suitable anatomy (Class IIA indication). The utility of EVAR for those at low or moderate surgical risk is uncertain (Class IIB indication) (11). Notably, these guidelines were developed prior to the publication of three major randomized trials summarized below (Table 35-1).
The Dutch Randomized Endovascular Aneurysm Repair trial (DREAM) was a randomized trial comparing EVAR with open repair in 351 patients with AAA >5 cm. After a mean follow-up of 6.4 years, survival was similar in the EVAR and open-repair groups (68.9% vs. 69.9%, p = 0.97). Freedom from reintervention was lower in the open-repair group (70.4% vs. 81.9%, p = 0.03) (12).
The United Kingdom Endovascular Aneurysm Repair trial (EVAR 1) randomized 1,252 patients with AAA >5.5 cm to open repair or EVAR. Thirty-day mortality was lower with EVAR (1.8% vs. 4.3%, p = 0.02), but by 8 years of follow-up, mortality rates were similar (HR: 1.03; 95% CI: 0.86-1.23) (13). The United Kingdom Endovascular Aneurysm Repair trial (EVAR 2) randomized 404 patients considered inoperable for open surgical repair to EVAR versus no repair. Procedural mortality associated with EVAR was 7.3%. During 4 years of follow-up, while aneurysm-related mortality was reduced in the EVAR group, all-cause mortality was similar between groups. Over one quarter (27%) of EVAR patients required reintervention by 6 years, and health care expenditures were substantially higher in the EVAR group (14).
LOWER EXTREMITY
General Overview
While lower extremity PAD is a highly prevalent condition affecting 14.5% of the population older than 70 years (15), the majority of patients with PAD are asymptomatic, and most will not develop limb-threatening ischemia over time. Additionally, in symptomatic individuals, natural history studies suggest that most (70%—80%) will have stable claudication symptoms and that, fortunately, <4% will require amputation during long-term follow-up (16). In light of these principles, percutaneous revascularization of the lower extremities is commonly reserved for the treatment of lifestyle-limiting symptoms that have persisted despite conservative measures, and that of limb-threatening ischemia, namely rest pain or tissue loss. Conservative management includes hygienic and supportive measures to prevent skin breakdown and infection, exercise conditioning, pharmacotherapy for claudication, and—most importantly—modification of risk factors to reduce the profound associated cardiovascular morbidity: smoking cessation, cholesterol reduction, treatment of diabetes, antihypertensive therapy, and antiplatelet therapy (11).
Lower extremity PAD commonly involves multiple vascular segments. As a general principle, inflow disease (i.e., the more proximal segments) should be treated first, or in concert with treatment of the more distal vascular beds (17).
Iliac Interventions
The TransAtlantic Inter-Society Consensus (TASC) document classification was developed to categorize aortoiliac lesions and guide therapy (Table 35-2) (18). In the TASC document, endovascular therapy was recommended for TASC A and B lesions, surgery
was recommended for TASC D lesions, and individually tailored decisions were considered for TASC C lesions. While the TASC classification system provided a simple schema to categorize lesion complexity, the evolution of endovascular technology and the advancement of techniques have permitted access to increasingly complex patients and lesions, and have rendered the guidelines expressed in this landmark document less relevant to contemporary clinical practice. In the current era, endovascular approaches are considered for most lesion subsets in most patients, weighing the relative risk-benefit profile, likelihood of successful and durable outcome, and patient preference when considering relative merits compared with surgical approaches. In the CLEVER trial, 111 patients with symptomatic aortoiliac stenosis were randomized to treatment with a supervised exercise program, stenting, or optimal medical therapy. The primary endpoint, improvement in peak walking time at 6 months, was highest in those who underwent a supervised exercise program (5.8 ± 4.6 minutes greater than baseline), slightly lower for those treated with stenting (3.7 ± 4.9), and lowest for those randomized to treatment with optimal medical care (1.2 ± 2.6). Of interest, a secondary endpoint, including improvement in quality of life, was achieved in a greater number of patients treated with stenting than with those who underwent an exercise program, calling into question the generalizability and application to clinical practice of the study’s primary finding (19).
was recommended for TASC D lesions, and individually tailored decisions were considered for TASC C lesions. While the TASC classification system provided a simple schema to categorize lesion complexity, the evolution of endovascular technology and the advancement of techniques have permitted access to increasingly complex patients and lesions, and have rendered the guidelines expressed in this landmark document less relevant to contemporary clinical practice. In the current era, endovascular approaches are considered for most lesion subsets in most patients, weighing the relative risk-benefit profile, likelihood of successful and durable outcome, and patient preference when considering relative merits compared with surgical approaches. In the CLEVER trial, 111 patients with symptomatic aortoiliac stenosis were randomized to treatment with a supervised exercise program, stenting, or optimal medical therapy. The primary endpoint, improvement in peak walking time at 6 months, was highest in those who underwent a supervised exercise program (5.8 ± 4.6 minutes greater than baseline), slightly lower for those treated with stenting (3.7 ± 4.9), and lowest for those randomized to treatment with optimal medical care (1.2 ± 2.6). Of interest, a secondary endpoint, including improvement in quality of life, was achieved in a greater number of patients treated with stenting than with those who underwent an exercise program, calling into question the generalizability and application to clinical practice of the study’s primary finding (19).
TABLE 35-1 Modern Randomized Trials of EVAR | |
|---|---|
|