Chapter 137
Thoracic and Thoracoabdominal Aneurysms
Branched and Fenestrated Endograft Treatment
Tara M. Mastracci, Roy K. Greenberg
Based on a chapter in the seventh edition by Roy K. Greenberg and Lars G. Svensson
In memory of Roy K. Greenberg, MD (1964-2013), who’s dedication to learning and innovation inspired us all. His contributions to the treatment of complex aortic disease, as reflected in this chapter, have saved the lives of many patients.
Open and endovascular treatment of thoracic and thoracoabdominal aneurysm has been discussed in detail in Chapters 134 and 135, including hybrid debranching repair. Advancements in branched and fenestrated devices now make pure endovascular thoracoabdominal aortic repair a possibility, and they have evolved since the early experience reported in 1999.1,2 Development of a minimally invasive option for treatment of complex aneurysm disease was imperative because many patients presented with multiple comorbidities and in the setting of previous aortic operations, making open repair very high risk. Today, the use of complex aortic devices to treat primary and reoperative aortic aneurysm or dissection is becoming increasingly more commonplace and in some jurisdictions has become the standard of care.
History
Fenestrated endografts were pioneered in a canine model and developed to extend the area suitable for sealing and fixation (proximal “landing zone”) for infrarenal aortic aneurysms with short necks.1,2 Once it was established that placement of fenestrations and branch stent grafts did not jeopardize the integrity of the proximal sealing zone or incorporated arteries, clinicians began to extend repairs to more complex aneurysms, until complete thoracoabdominal repairs were possible. Initially fenestrations were mated with uncovered balloon-expandable stents, but it was soon found that the durability of the repair was improved if covered stent-grafts were used.3 At the same time, the behavior of stent-grafts mated to fenestrations was being observed over time. It became quickly evident that fenestrations alone were not adequate to deal with extensive aneurysms in which the luminal diameter within the visceral segment of the aorta was much larger than the stent-graft. Thus, the concept of a side-arm branched graft, in which a cuff of fabric is attached to the main body of the device, thereby providing a longer landing zone, was developed. This development allowed for the use of self-expanding stents that could be oriented either axially or directionally toward the target visceral vessel. These branches were initially evaluated in the internal iliac arteries and then extended to use in the celiac and superior mesenteric and renal arteries. Over time, intercomponent movement of main body components was observed within large aneurysm sacs. Device design was changed to maximize intercomponent overlap.4 Emphasis was placed on the creation of a design that allows intercomponent movement to continue in at least one region of the repair with the use of increased overlap, so that other intercomponent joints could be affixed with barbs. Such a design helps remove stress from the proximal fixation zone, theoretically diminishing the risk of losing branch vessels because of migration.
Device Configurations
Visceral/Renal Aorta
Fenestrated Stent-Grafts
Fenestrated devices can be used to treat juxtarenal aneurysms, with covered stents used for the renal and superior mesenteric position (see Chapter 91). Several different juxtarenal fenestrated devices are available, at different stages of development (Fig. 137-1). The most common initial configuration consisted of two renal fenestrations and a scallop for the superior mesenteric artery (SMA). As more experience was achieved, the seal zone was moved more proximally, so the number of incorporated visceral vessels in the repair increased. For custom-made devices the number of vessels is not an issue, because the location of fenestrations can be measured accurately and placed according to the patient’s relative anatomy. However, there is a growing emphasis on off-the-shelf devices, in which the designs are intended to accommodate some variability in patient anatomy by modifying the manner in which the vessels are incorporated. Thus, larger scallops for the SMA and self-orienting fenestrations for the renal arteries are being introduced (see Fig. 137-1). In all cases, the fenestrated main body is intended to be mated to a standard, more distal bifurcated component extending to the common iliac arteries.
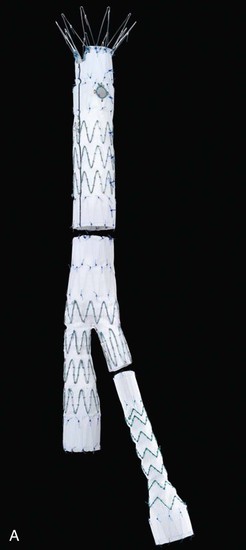


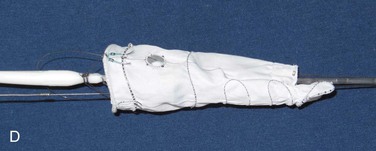
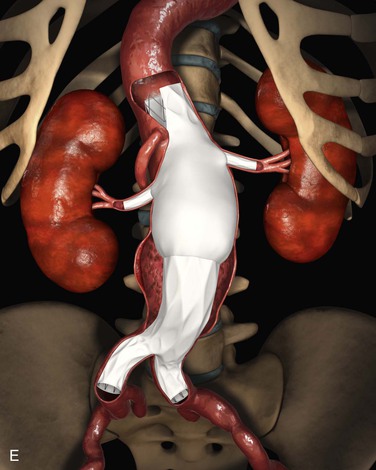
Figure 137-1 Fenestrated devices available at the time of publication. A, Zenith AAA Fenestrated System (Cook Medical, Inc., Bloomington, Ind). B, Medtronic, Inc. (Minneapolis, Minn), device. C, Zenith P-Branch off-the-shelf fenestrated system (Cook Medical). D, Anaconda System, courtesy Peter Bungay. E, Endologix, Inc. (Irvine, Calif), Ventana Fenestrated System (still investigational in United States).
The device with the most clinical use to date is the custom-made Cook Zenith fenestrated device (Cook Medical, Inc., Bloomington, Ind) (Fig. 137-2). It has reinforced fenestrations with nitinol rings and gold markers to help with fluoroscopic orientation. There is an uncovered top stent with barbs to aid with fixation, and the first two proximal stents of the main body are intended to provide a circumferential seal. This main body, which is a tube, is subsequently mated to a more distal bifurcated component and iliac limbs to improve customizability. The entire device is constructed with stainless steel or nitinol Gianturco Z stents and Dacron fabric. It is attached to a nitinol cannula and loaded into a flexible hydrophilic sheath. A staged delivery is performed with the use of trigger wires, allowing for device unsheathing while it remains attached to the delivery system and partially constrained with diameter-reducing ties. This arrangement provides a means to manipulate the device (longitudinally and radially) within the aorta in order to optimize orientation.


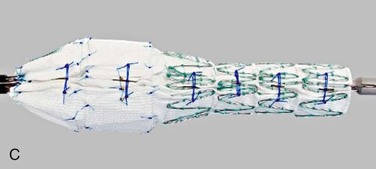
Figure 137-2 Details of a fenestrated device. A, Nitinol-reinforced “scallop,” with three gold radiopaque markers. B, Small fenestration with reinforced nitinol ring and four gold radiopaque markers. C, Diameter-reducing ties that constrain the device and allow for manipulation prior to complete deployment.
Branched Stent-Grafts
Branched stent-grafts are manufactured with branches preattached to the main body. The majority of experience with branched visceral devices comes from custom-made devices using Cook Medical’s Zenith platform. Both straight cuffs and helical limbs have been used effectively to bridge the aneurysmal aorta into the celiac and SMA. In some branched grafts, directional cuffs are also used to stent the renal arteries (Fig. 137-3). Despite minor differences in configuration, the the two devices are generally the same in construction: They are custom designed to fit patient anatomy, created with Dacron fabric over stents, have fabric side-arm branches that may or may not be reinforced with nitinol, and are loaded onto a delivery system that affixes an implant as described previously, optionally in a “preloaded” fashion. The significance of the preloaded delivery system is substantial (Fig. 137-4). If a device is “preloaded,” a catheter is threaded through the delivery system and then through the branch, exiting at the proximal aspect of the delivery system. This is utilized in all iliac branch devices (IBD)s, with the Pivot Branch (Cook Medical) and Ventana (Endologix, Irvine, Calif) devices, and selectively in visceral or supraaortic trunk branched devices. For a branched device, when a wire is threaded through the catheter, it can be snared from above, allowing direct access into the limb without the need for cannulation. This feature decreases intraoperative implantation times significantly and, most importantly, provides a stable platform over which sheaths and subsequent mating devices can be delivered, even through the most tortuous arch anatomy.


Figure 137-3 Visceral artery branched devices, showing both straight cuffs (A) and helical branches (B).
Iliac Arteries
Internal iliac branched grafts are the simplest configuration of side-arm branch graft technology, because they involve only a single branch. They are intended to treat common iliac aneurysms; however, the branched configuration is also being considered for treatment of extremely short common iliac arteries in infrarenal aneurysms, in which traditional iliac limbs may provide inadequate seal, and for patients with aneurysms in the common trunk of the internal iliac artery itself.
One manufacturer, Cook Medical, has devices available at the time this chapter is being written, and a second, W.L. Gore & Associates (Flagstaff, Ariz) has a device near the end of development (Fig. 137-5). In this configuration, a cuff of graft material is attached to the mid-portion of an iliac limb to allow access to the internal iliac artery (IIA) and the distal portion of the limb still seals within the external iliac artery. A covered stent is intended to be mated to the side-arm branch. There remains controversy whether this is optimally a balloon-expandable or self-expanding construct. In either case, these branch stents can join the iliac limb with the IIA by landing within either the main trunk of the IIA or the posterior or anterior branch, depending on the morphology of the IIA. These mating stents are commonly brought up and over the aortic bifurcation but can also be placed from an upper extremity access site.

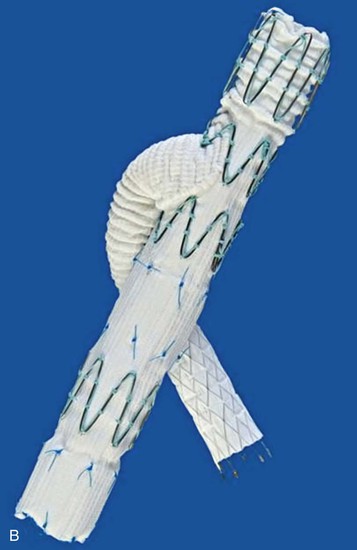

Figure 137-5 Details of iliac artery branched devices. A, Gore Medical internal iliac branched device (W.L. Gore & Associates, Inc., Newark, Del). B, Cook Medical, Inc. (Bloomington, Ind) Zenith hypogastic branch device; this is the helical branch version. C, Cook Medical bifurcated-bifurcated device.
Arch and Ascending Aorta
There is substantial four-dimensional movement through the cardiac cycle that makes accurate placement of an aortic stent graft in the ascending and arch aorta a greater challenge. Further, the proximal restriction of both coronary arteries and aortic valve affect the planning of a useful delivery system. Finally, a healthy proximal landing zone is required that mandates that the ascending aorta either is replaced with surgical graft material or has an acceptable diameter and appearance on imaging studies.
The use of fenestrations for arch vessels has been described, but there are no large published series of experience with them. Yet, this technique is a direct extension of abdominal aortic fenestrations5 with a different set of challenges. The alignment of fenestrations in the abdominal segment is aided by the relatively straight morphology of the visceral aorta and the ability to rotate the device while it is still attached to the delivery system. This is not possible in the arch. Consequently, alternative techniques have been employed to orient fenestrations. Methods have included the use of precurved delivery systems, preloaded catheters and wires, and torque-controlled flexible devices (Fig. 137-6). Mating stents or stent-grafts are typically placed via brachial or axillary artery access, possibly necessitating the placement of a carotid-subclavian bypass graft, depending on the target vessels. The concept of fenestrating a stent-graft using a retrograde approach has been termed in situ fenestration. Although this procedure has been described in animal studies6 and a few human cases, it is not commonly performed.7





Figure 137-6 Arch branch devices. A and B, Cook Medical, Inc. (Bloomington, Ind) arch branch device. C, W. L. Gore & Associates, Inc. (Newark, Del) thoracic device with branch. D and E, Bolton Medical, Inc. (Sunrise, Fla) arch branch device.
There are published series describing fenestrated aortic arch devices, and the available data largely come in the form of case reports.8 The Najuta endograft system originated from the Tokyo Medical University.9 Several geometric shapes of the graft exist and are tailored to fit the individual patient arch anatomy. The device is constructed with longitudinally connected Z stents covered with expanded polytetrafluoroethylene (ePTFE) that is sutured only at the proximal and distal ends of the device. The device has been used in hundreds of patients, yet there are no peer-reviewed published reports in the English literature.
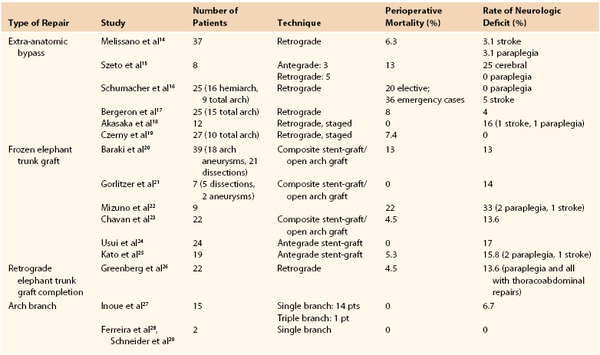
The first successful deployment of a branched graft for the supra-aortic trunk vessels was reported by Inoue et al10 in 1996, and the group’s data were supplemented in 1999.11 Outcomes for arch repair are shown in Table 137-1. The device utilized consisted of a unibody graft with multiple (up to three) limbs that are snared and pulled into each of the aortic trunk vessels. Surprisingly, this procedure was accomplished with use of local anesthesia in 14 of the first 15 patients treated. Issues including stroke risk and graft material durability were encountered with the development of these devices, which have slowed the refinement of the device and technique considerably. Chuter et al12 reported successful treatment of an arch aneurysm using the first modular design in 2003. This device is functionally a bifurcated endograft that is deployed from a conduit sewn to the innominate or proximal right carotid artery. The proximal portion of the implant resides within the mid-ascending aorta, and the ipsilateral limb is deployed into the innominate artery. Following proximal component deployment, cannulation of the “contra-lateral limb” is obtained from a retrograde femoral access site, and the distal thoracic device is inserted into the overlap segment and deployed. Two reports of this technique have been published.12,13 However, technical problems and complications rendered this device obsolete as well.
The latest advancements in endovascular treatment for aortic arch disease are branched graft that have internal docking limbs (see Fig. 137-6). The device that has been used the most to date is Cook Medical’s Arch Branch graft. It requires 5 cm of normal-caliber proximal landing zone in the ascending aorta, implying freedom from ascending disease, or a previous interposition graft repair. At the time of publication, 40 devices have been implanted (Drs. K. Ivancev and S. Haulon, personal communication, 2013). As experience is gained with this device, the indications for use will be further refined. Bolton Medical, Inc. (Sunrise, Fla) has also reported some successful cases with a single-branch arch device, and descriptions from other manufacturers have been disclosed and are published, yet none has been implanted in a human as yet.
Physician-Modified Stent-Grafts
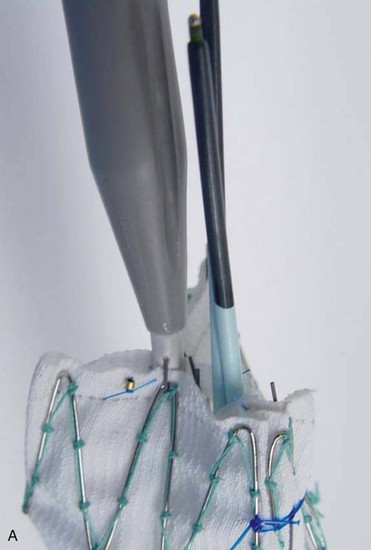
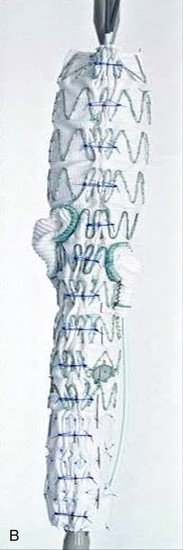
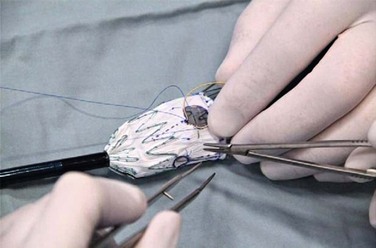
A criticism of custom-made branched and fenestrated stent grafts is that there is a significant manufacturing delay, which prohibits their use in urgent or emergency situations. Some physicians have chosen to modify infrarenal or thoracic devices by adding branches and fenestrations on the “back table” of the operating room in order to offer this option to patients who need urgent treatment. Early results have been described by different groups,30–32 and Oderich et al32 have reviewed methods for performing the procedures. Devices are created with reinforced fenestrations or branches constructed from portions of self-expanding stent grafts, depending on the configuration required (Fig. 137-7). The largest reported series to date involved 47 consecutive patients, with a perioperative mortality of 2% and a technical success rate of 98%.30 Long-term outcomes are not yet available. Such procedures assume a great deal of responsibility for the implanting physician unless performed in the context of a formal investigational device exemption study within the United States.
Preoperative Planning and Sizing
Aortic imaging with a high-resolution computed tomography (CT) scan should be obtained in all patients without contraindications to a moderate dose of contrast agent. A CT scan of the entire aorta allows a clinician to assess concomitant pathology in other aortic distributions and determine the relative anatomy of branch vessels. The CT scan should be reconstructed at intervals no more than 1 mm, to offer the best resolution for visualizing all pathology. If arch pathology is suspected, a CT scan can acquire data in an electrocardiography (ECG)–gated fashion, and retrospective gating can be used to provide clear images of the highly mobile proximal aorta. It is now commonplace to use post-processing software to manipulate the Digital Imaging and Communications in Medicine (DICOM) data in three dimensions, a procedure that has become increasingly more accurate as isovoxel data (data that are equivalent in the x, y and z axis) are now routinely obtained. This step allows for the creation of a center line of flow (CLF) projection that is the workhorse of planning and sizing for devices with fenestrations or branches. The center line of flow view aids in assessing the proximal landing zone, which should reside within parallel-walled, relatively nontortuous, atheroma-free aorta regardless of proximity to branch vessels. Such a landing zone ensures that sealing and fixation will occur in portions of the aorta that are least prone to further degeneration. Severe angulation, occlusive disease in access vessels, or extensive debris within the aortic lumen also must be assessed, because they are factors that may lead to difficulty with deployment, complications, or ultimate failure of the device.
Proximal Sealing Zone
Choice of the area suitable for sealing in the proximal portion of the aorta (proximal sealing zone) is one of the most important decisions to be made in the planning of a fenestrated/branched device procedure. Aorta that is not healthy is “vulnerable” to aneurysmal expansion, potentially destabilizing the entire repair. The introduction of complex devices has removed the constraints of branch vessel locations for determining the proximal seal zone, because all branches can be incorporated into a repair. The choice of sealing zone should be carefully considered, since compromising the sealing zone by incorporating fewer branches will lead to increased endoleaks. Given the difficulty of re-intervening for a type I endoleak in a branched segment, it is extremely important to ensure that the sealing zone is stable and durable.
Fixation
The stent-graft is usually fixed to the aorta with barbs on either uncovered or covered struts. It is common to use an uncovered stent with barbs in the infradiaphragmatic aorta. In the thoracic aorta, covered stents with barbs are preferred because of the tortuosity of this segment and the need to maximize sealing. When possible, sealing and fixation should occur in a straight segment of aorta. Maximizing overlap rather than including barbs, in order to allow for some intercomponent movement as the device adjusts within a remodeling aneurysm, usually provides fixation between devices, particularly in the infrarenal aorta. Proximal intercomponent joints are barb reinforced when possible.
Patient Considerations
Overall, the device plan must consider the lifespan of the patient, the nature of the disease (genetic vs. degenerative), the risk of paraplegia based on the presence of hypogastric and vertebral territories, and previous aortic procedures. Although it is common to use branches instead of fenestrations in the crossing of large aneurysms, there are some exceptions. For example, the configuration commonly seen in patch aneurysms after a remote thoracoabdominal aneurysm repair changes the projection of the visceral branches, making access through a caudally directed branch extremely challenging, but suits a fenestration quite well. Another obvious example would be the presence of an enlarged false lumen with a very small true lumen in a chronic dissection: in this case again, a fenestrated device would be preferable despite the fact there is a large aneurysm at the level of the branches. In general care should be taken to preserve all access vessels and all territories supplying blood to the spinal cord because it is difficult to predict the future needs of the patient. Additional consideration should be given to the potential benefit of a staged procedure for extensive aneurysms. On the basis of evidence from Bischoff et al,33 spinal outcomes may benefit from a staged approach to total coverage of intercostal arteries. Thus, when possible, placing a thoracic segment first and following up months later with a fenestrated or branched segment may decrease the risk of spinal cord injury. In some centers, this same staging is achieved by leaving an additional “perfusion branch” open to supply blood to the aneurysm sac, a technique popularized by Krassi Ivancev. There exists an added risk of rupture during the interim, but the concept is well supported by both human and animal data (described later).
Consideration of the patient’s age and overall health status, as well as the presence of contiguous aneurysms, is important in the sizing of fenestrated endografts. For example, an 88-year-old patient with a juxtarenal aneurysm would likely do well with a three-vessel fenestrated graft with a single scallop to the celiac artery. However, a 69-year-old patient with the same juxtarenal configuration and in whom an area of proximal descending thoracic aorta is greater than 3.5 cm may be better suited with a graft that extends above the celiac artery so that subsequent thoracic repairs can be connected to this visceral segment, providing a modular repair for the aorta if it grows through the years. However, this plan must be balanced against any increased risk of spinal cord injury incurred by increasing the amount of aortic coverage.
Aortic Arch Considerations
The realm of arch disease is new to the vascular surgeon, but as devices become more widely available to deal with the supra-aortic trunk vessels, it will become necessary to include these patients in the population routinely considered for vascular assessment. Currently, treatment is recommended for patients with arch aneurysms that exceed 5.5 to 6 cm, depending on the lesion morphology, etiology, and the extent of comorbid conditions. Patients with chronic arch dissection or with Marfan’s syndrome may require earlier intervention.34 Dissections originating within the aortic arch may be treated surgically in candidates who have good surgical risk or may be handled like uncomplicated distal dissections. Symptomatic penetrating ulcers and large ulcers (>2 cm) are best repaired in patients without extensive comorbid conditions. Owing to the novelty of the devices described, fenestrated and branched repair for aortic arch is largely reserved for patients without good options for open surgical repair, but endovascular treatment is expected to expand in this area as new arch-specific devices become available.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree