Chapter 30 Thoracic and Lumbar Sympathectomy
Indications, Technique, and Results
Minimally invasive endoscopic technology has rapidly transformed both thoracic and abdominal surgery and the field of surgical sympathectomies was no exception. For thoracic sympathectomy, the thoracoscopic procedure is used almost exclusively.1–3 Laparoscopic4 and retroperitoneoscopic techniques5,6 have also been developed for ablation of the lumbar sympathetic chain. Because of its relatively less invasive nature, the open technique for lumbar sympathectomy is still performed, but with rapidly decreasing frequency.7,8 Indications for both thoracic and lumbar sympathectomies have changed during the past decades. Earlier indications such as claudication, uncomplicated primary Raynaud syndrome, or scleroderma are not used.9 Refractory palmar and plantar hyperhidrosis, chronic pain syndrome, Buerger disease, frostbite, and complicated primary Raynaud syndrome and secondary Raynaud phenomenon with nonhealing digital ulcerations have become the most common indications for endoscopic sympathectomy.2,8,10
Historical Background
Although Jabouley suggested sympathetic denervation for vasospastic disorders as early as 1899, periarterial sympathectomy was introduced by Leriche only in 1913 for ischemic lesions caused by vasospasm.11 The first lumbar sympathectomy was performed by Royle in 1923 to treat a patient with spastic paralysis of the lower limb.12 The concept of sympathectomy was soon adopted by Adson and Brown,13 who performed lumbar sympathectomy to relieve vasospasm of the lower extremities. Adson and Brown14 were the first to perform cervicothoracic sympathectomy in 1929.
Using the single-scope technique, thoracoscopic sympathectomy was popularized by Kux15 in Austria as early as 1954. With the advent of endoscopic surgery, both thoracoscopic1–3 and laparoscopic or retroperitoneoscopic procedures5,6 have been developed for sympathetic denervation.
Anatomy and Physiology
Anatomy
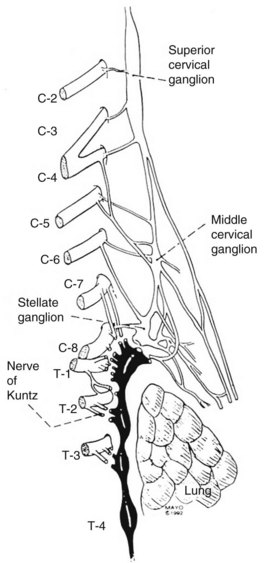
The sympathetic nervous system consists of the central autonomic network, which includes the brainstem, diencephalons, and cortex, and of the peripheral sympathetic pathways. The peripheral sympathetic pathway consists of preganglionic and postganglionic neurons. Information from the brainstem and hypothalamus descends through the lateral funiculus of the spinal cord to the preganglionic sympathetic fibers. The preganglionic sympathetic neurons originate in the anteromedial column of the thoracolumbar cord, between T1 and L2. These myelinated white nerve fibers travel in the ventral root of the spinal cord to the paravertebral sympathetic ganglia, where they synapse onto the postganglionic unmyelinated grey fibers. It is likely that each preganglionic axon innervates about ten postganglionic neurons.16 The postganglionic axons can leave the parasympathetic ganglia at the level of the synapse or can travel first up or down in the sympathetic chain before exiting the ganglion via grey rami. The regional activity of the sympathetic chain is the product of reflex arcs between somatic afferent fibers and preganglionic efferent fibers. For sympathetic denervation of the upper limbs, the interruption of the sympathetic chain from T2 to T4 is required. Although the stellate ganglion has some innervation to the upper limbs, resection of the stellate ganglion results in Horner syndrome (ptosis, myosis, and enophthalmos). Because of this associated morbidity, the stellate ganglion is not removed surgically. The nerves of Kuntz (Figure 30-1) are important because they provide direct collaterals from the T2 and T3 ganglia to the upper limbs. Resection of T2 and T3 ganglia (sympathectomy) is thought to be essential to achieve good and durable sympathetic denervation of the arms. The preganglionic fibers may bypass the paravertebral ganglia to synapse with more distal intermediate ganglia or may cross over to innervate the contralateral side as well. Therefore, as mentioned previously, a complete sympathectomy includes division of the preganglionic fibers and excision of the relay ganglia T2 and T3 and the intercommunicating fibers (the nerve of Kuntz). Several authors advocate T4 and T5 resection, especially in patients who undergo the operation for axillary hyperhidrosis.3
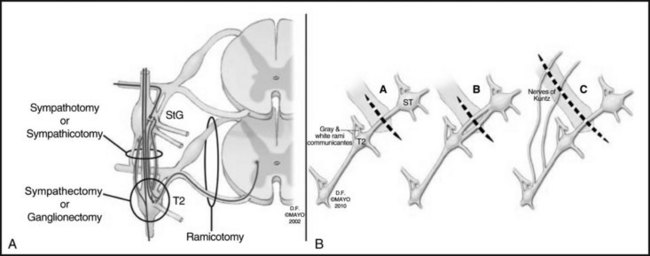
Figure 30-2 depicts the finer anatomy of the nerves and illustrates the difference between T2 sympathectomy and T1-T2 sympathotomy at the level of the second rib. Atkinson and colleagues2 advocate performing sympathotomy by severing all visualized sympathetic branches between the T1 (and C8) ganglion and the T2 ganglion to minimize axonal and potential neuron injury that may increase side-effects, particularly compensatory hyperhidrosis after sympathectomy.2
Physiology
The sympathetic nervous system modifies basal organ functions and mediates the body’s response to stress (“fright, flight and fight response”). The primary function of the peripheral sympathetic nervous system is to prevent heat loss by reducing the blood flow to skin and subcutaneous tissue of the limbs. The increased activity of the postganglionic sympathetic nerves, mediated by norepinephrine, results in decreased blood flow because of stimulation of the vasoconstrictor fibers innervating the blood vessels. There is increased sweating caused by stimulation of the sudomotor fibers innervating the exocrine glands (an activity mediated by acetylcholine, adenosine and other neuropeptides), and there is piloerection mediated through the activity of the pilomotor fibers which innervate the erector pili muscles.16 The sympathetic system antagonizes the vasodilatory effect of the parasympathetic nerves on arterial resistance vessels, on the cutaneous precapillary sphincters, and on the capacitance venules.
Several authors have investigated the effect of sympathectomy on blood flow of the limbs. Cronenwett and colleagues17 found no increase of nutrient capillary perfusion as a result of sympathectomy. Using intradermal xenon clearance, Moore and Hall18 observed increased flow to the cutaneous capillary circulation. Rutherford and Valenta19 failed to confirm increased muscle flow as a result of sympathectomy. Dalessandri and colleagues,20 however, described increased collateral circulation in patients who underwent sympathectomy.
Thoracic Sympathectomy
Indications
In an earlier study from the Mayo Clinic, Lowell and colleagues9 reviewed indications for cervicothoracic sympathectomy in 68 patients. The most frequent indications were atheroembolism followed by Raynaud syndrome, causalgia, hand ischemia, reflex sympathetic dystrophy, and Buerger disease. The most frequently used indications for thoracic sympathectomy are medically refractory palmar-plantar hyperhidrosis and causalgia.2,3 Additional indications include distal digital occlusion, vasospastic disorders with digital ulcerations not responding to medical treatment, Buerger disease, and frostbite.1,10,21–23 There is a beneficial effect of sympathectomy on facial blushing.10 Uncomplicated Raynaud disease and scleroderma are contraindications to cervical sympathectomy (Table 30-1).
TABLE 30-1 Indications for Sympathectomy
| Indication | Thoracic Sympathectomy | Lumbar Sympathectomy |
|---|---|---|
| Best | ||
| Acceptable | ||
| Contraindications | Uncomplicated Raynaud disease |
Surgical Technique
Open Surgical Techniques
Transaxillary Approach.
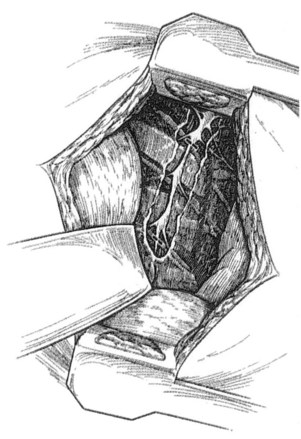
Patients who undergo transaxillary approach to the thoracic sympathetic chain are placed into a 90-degree lateral decubitus position with the arm elevated by the surgical assistant or with the help of an arm board. The transverse skin incision is performed in the axillary fossa, just distal to the axillary hairline. The dissection is carried down to the third rib, avoiding injury to the long thoracic nerve anteriorly and the subscapular artery and thoracodorsal nerve posteriorly. The pectoralis major muscle is retracted anteriorly, and the latissimus dorsi is retracted posteriorly. A 6-cm–long segment of the third rib is dissected from its periosteum, and it is removed. The pleura is entered, and the lumbar sympathectomy chain is dissected in the paravertebral region posteriorly by incising the posterior pleura (Figure 30-3). The chain is located 2-3 cm centimeters lateral to the azygos vein. The chain between T1 and T5 is sharply dissected, and the sympathetic chain with T2-T3 and usually T4 ganglia is removed. The stellate ganglion should not be removed, and dissection toward the neck should be avoided.
Cervical Approach.
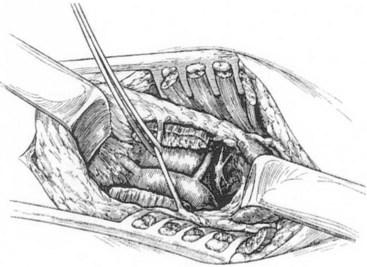
The cervical approach is through a supraclavicular skin incision. A 6-cm–long transverse incision is made 2 cm proximal to the clavicle, and the posterior belly of the sternocleidomastoid muscle is divided. The external jugular vein is ligated and divided, and the anterior scalene muscle is divided, carefully retracting the phrenic nerve medially. The subclavian artery is gently retracted caudally, and the stellate ganglion is exposed medial and anterior to the brachial plexus. It usually lies immediately lateral to the vertebral artery (Figure 30-4). The stellate ganglion is preserved, and dissection is carried down into the thoracic cavity, retracting the pleural dome as much as possible. The T1, T2, and T3 ganglions can usually be removed through this approach. On the left side, the dissection is similar, but attention must be paid to avoid injury to the thoracic duct. If injury to the thoracic duct or to one of the larger cervical ducts and leak of chyle is identified, ligation of the duct is warranted to avoid the complications of a chylocutaneous cyst or fistula.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree