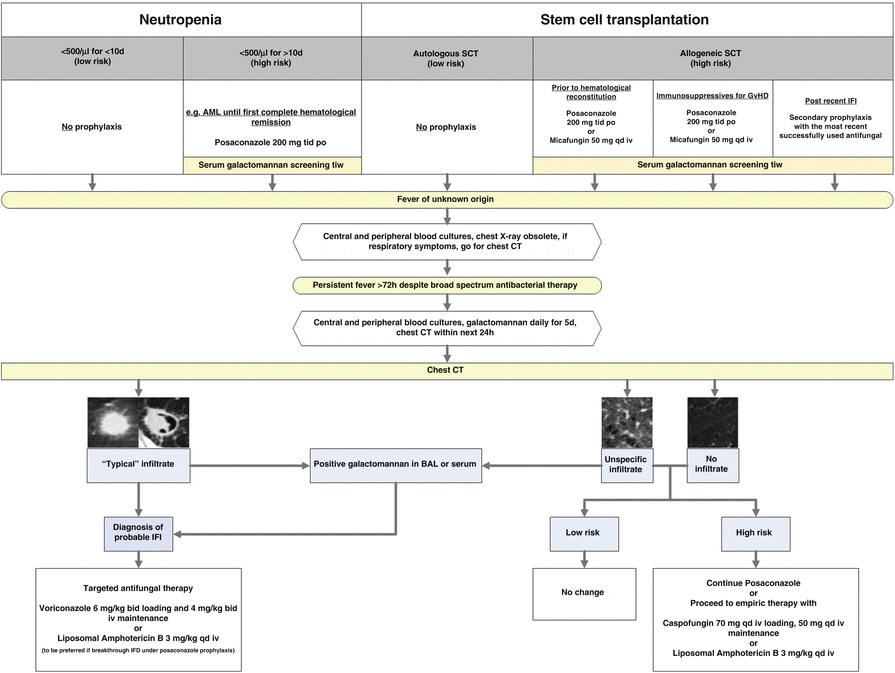
Fig. 14.1.
Antifungal treatment strategies for patients at high risk for fungal infection [Taken from Ruping et al. (2008)]
In clinical practice, these situations may often overlap, making an exact classification difficult. However, the implied categorization of the probability of IFD can be of considerable help in the evaluation and choice of antifungal treatment for particular patients and entire patient populations.
II. Prophylaxis
IFD have a profoundly negative impact on patient survival (Gudlaugsson et al. 2003). If treatment is delayed, mortality increases even further (Morrell et al. 2005; Garey et al. 2006). This is a severe clinical problem in the context of unreliable diagnostic tools, such that the concept of prophylaxis becomes attractive.
Prophylactic strategies follow the a priori risk assessment of a patient, or – more likely – a patient group because prophylaxis should be an institutional policy rather than a case-by-case decision.
Classical immunosuppressed patient groups, i.e., long-lasting neutropenia and allogeneic hematopoietic stem cell transplant (HSCT) recipients, need a different approach to patients in intensive care or undergoing abdominal surgery.
A. Neutropenia and Hematopoietic Stem Cell Transplantation
Neutropenic patients and hematopoietic stem cell recipients have been treated prophylactically in about 100 clinical trials (Cornely et al. 2003). Some of these trials showed convincing benefits. In the mid-1990s, fluconazole was shown to improve survival in stem cell transplantation (Slavin et al. 1995; Marr et al. 2000). It almost instantly became standard in many transplant protocols, although lacking activity against filamentous fungi, specifically aspergillosis. The logical next step were randomized comparisons of fluconazole versus itraconazole. In stem cell transplantation, itraconazole improved protection from aspergillosis, but did not lead to a further increase in survival rates. Approximately one third of the patients receiving itraconazole had gastrointestinal adverse effects and were finally unable to tolerate this prophylaxis (Marr et al. 2004b). Micafungin proved to be as effective as fluconazole in the hematopoietic transplant setting (van Burik et al. 2004). Posaconazole was evaluated in a randomized clinical trial in patients immunosuppressed for graft-versus-host disease. Numerically more fungal infections were diagnosed in patients receiving the comparator drug fluconazole, although this difference did not reach statistical significance (Ullmann et al. 2007). In neutropenic patients undergoing chemotherapy for remission induction in acute myelogenous leukemia, posaconazole reduced the incidence rate of invasive fungal infection to a mere 2 %, whereas the comparators fluconazole and itraconazole resulted in a rate of 8 %. This difference had a profound impact on 100-day survival (Cornely et al. 2007b). The patient numbers needed to treat to prevent a fungal infection and to prevent a death until day 100 were 16 and 14, respectively, which has been considered low (Cornely and Ullmann 2008). Posaconazole became the standard approach for the above populations.
Other trials compared voriconazole and fluconazole, and voriconazole and itraconazole. Although no benefit of voriconazole over these comparators was demonstrated, voriconazole was non-inferior and better tolerable than itraconazole (Wingard et al. 2010; Marks et al. 2011). Table 14.1 summarizes our recommendations on the use of antifungal prophylaxis in the neutropenic and/or transplanted patient.
Table 14.1
Prophylaxis and treatment of IFD in neutropenic patients
Strategy
Underlying condition
Risk factor
Antifungal
Dose
Reference
Prophylaxis
Acute leukemia or myelodysplastic syndrome
Induction therapy
Posaconazole
200 mg tid po
Cornely et al. (2007b)
Allogeneic SCT
Neutropenia and immunosuppression
Posaconazole
200 mg tid po
Micafungina
50 mg qd iv
van Burik et al. (2004)
Empiric treatment
Total duration of neutropenia >10 days
Persistent fever for >72–96 h
Caspofungin
70 mg loading dose and 50 mg qd iv
Walsh et al. (2004)
Liposomal amphotericin B
3 mg/kg qd iv
Walsh et al. (1999)
Pre-emptive treatment
Total duration of neutropenia >10 days or prior allogeneic SCT
Laboratory or radiographic results conclusive of IFD
Unknown
NA
Targeted treatmentb
Any
Any
Voriconazolec
6 mg/kg bid loading dose, followed by 4 mg/kg bid iv
Herbrecht et al. (2002)
Liposomal amphotericin B
3 mg/kg qd iv
Cornely et al. (2007a)
B. Intensive Care
In contrast to the patient populations mentioned above, the number of clinical trials in intensive care unit (ICU) populations is very limited. One likely reason is the heterogeneity of patient groups. Today, the patients groups appropriate for prophylaxis have not been defined.
Many trials have aimed to identify risk factors that might enable physicians to predict occurrence of IC; however, none of these risk scores has made its way into clinical practice, so far (Michalopoulos et al. 2003; Ostrosky-Zeichner et al. 2007; Dupont et al. 2003; Shorr et al. 2009; Paphitou et al. 2005). The most promising approach evaluated fluconazole in a placebo-controlled clinical trial in a population of patients who had undergone recent abdominal surgery and who had recurrent gastrointestinal perforations or anastomotic leakages. Intraabdominal candidiasis occurred significantly less often with fluconazole prophylaxis (Eggimann et al. 1999). The study was well designed, and the population was successfully predicted to be at high risk. However, the study was too small to warrant a strong recommendation (Cornely et al. 2012). A larger trial evaluated the comparison in a population of surgical patients with an expected ICU stay of ≥3 days. Time to fungal infection was significantly longer under fluconazole prophylaxis (Pelz et al. 2001). From today’s perspective, the study used outdated definitions of invasive fungal infection and was conducted in a setting of unusually high Candida infection incidence rates. This might have prevented the study from leading to widespread use of fluconazole in this indication (Pelz et al. 2001).
Meta-analyses found fluconazole at 400 mg/day to be superior to placebo in preventing invasive fungal infection in critically ill surgical patients, but could not identify a patient population appropriate for prophylaxis (Cruciani et al. 2005; Shorr et al. 2005; Playford et al. 2006a, b; Vardakas et al. 2006).
In contrast to trials in the hematological settings above, none of the clinical trials in ICU settings have led to reduced mortality.
III. Empiric Treatment
Most studies on empiric antifungal treatment have been performed in the setting of persistent febrile neutropenia. In this setting, amphotericin B deoxycholate (D-AmB) used to be considered the gold standard until it was challenged by three major randomized trials. All three trials used similar composite endpoints, including survival, defervescence during neutropenia, successful treatment of baseline IFD, prevention of breakthrough infections, and absence of early discontinuation due to side effects.
Initially, D-AmB and liposomal amphotericin B (L-AmB) were compared in a randomized, double blind, multicenter study. There was no difference in treatment success and survival rates between groups, but breakthrough infections and nephrotoxicity were observed less often in the L-AmB group. Thereafter, L-AmB was established as the new standard treatment in the setting of persistent febrile neutropenia (Walsh et al. 1999).
Voriconazole was compared to this new gold standard in a subsequent open-label, randomized, multicenter trial. Although breakthrough IFD and nephrotoxic events were significantly lower in the voriconazole arm, non-inferiority criteria for voriconazole were not met. In addition, voriconazole was associated with more episodes of transient visual changes and hallucinations (Walsh et al. 2002). Voriconazole was thus not established as an antifungal of choice in the empiric setting.
Finally, caspofungin and L-AmB were compared in a double-blind, multinational non-inferiority trial including 1,095 patients with hematological cancer. Overall success rates were similar in both treatment groups, and caspofungin fulfilled statistical criteria for non-inferiority. Furthermore, fewer premature treatment discontinuations due to adverse events were recorded in the caspofungin group (Abzug and Walsh 2004).
Table 14.1 summarizes the outcome of these trials with respect to current empiric treatment recommendations.
The current basis of evidence does not favor empiric antifungal therapy in non-neutropenic ICU patients with blood cultures negative for Candida spp. (Zilberberg et al. 2010). Some authors would classify patients with blood cultures positive for Candida spp., but pending information on species and susceptibility, as potential recipients of empiric treatment (Cruciani and Serpelloni 2008). However, we prefer to discuss these patients in the context of targeted treatment.
IV. Targeted and Pre-emptive Treatment
A. Invasive Aspergillosis
Three major groups are at high risk for contracting IA: patients with hematologic cancer, recipients of solid organ or stem cell transplants, and a heterogeneous group of otherwise immunosuppressed patients, e.g. by long-term corticosteroid administration, acquired immunodeficiency syndrome without antiretroviral therapy, chronic obstructive pulmonary disease (COPD), or liver failure. In patients outside these risk groups, occurrence of IA is unlikely and suggestive clinical signs and symptoms should be scrutinized to avoid initiation of unwarranted antifungal treatment. Even in patients at risk, a suspected diagnosis of IA should be consolidated by a thorough diagnostic workup. Nevertheless, many patients remain without proof of IFD such that treatment is initiated in a pre-emptive as opposed to a targeted treatment setting. This is also reflected in the design of clinical studies assessing the treatment of IA. Usually, they allow for the inclusion of patients in both pre-emptive and targeted settings. In this section, relevant results from these studies will be presented and discussed.
A milestone randomized, open-label clinical trial focused on immunocompromised patients undergoing allogeneic HSCT, autologous HSCT, or chemotherapy for acute leukemia. As a minimum diagnostic requirement for inclusion into the study and randomization to intravenous treatment with voriconazole or amphotericin B deoxycholate (D-AmB), patients needed to present with an infiltration considered typical of IA (halo or air-crescent sign). Presentation of further microbiological proof of IFD was encouraged, but not compulsory. Based on these criteria, 144 patients in the voriconazole arm and 133 patients in the D-AmB arm were included into the final efficacy analysis. Treatment success had been defined as complete or partial clinical and radiological response and was observed in 53 % and 32 % of patients in the voriconazole and D-AmB groups, respectively. At week 12, survival rates of 71 % and 58 %, respectively, were reported. Based on these findings, the primary endpoint, defined as the non-inferiority of voriconazole as compared with D-AmB at week 12, could be fulfilled. Furthermore, significantly fewer severe drug-related adverse events were observed in the voriconazole arm. This trial established voriconazole as the treatment of choice for IFD in the pre-emptive and targeted treatment setting (Herbrecht et al. 2002).
A consecutive multinational, double-blind trial compared the clinical efficacy of liposomal amphotericin B (L-AmB) 3 mg/kg qd iv (once daily, intravenously) or 10 mg/kg qd iv for 14 days, followed by 3 mg/kg qd iv. Concerning patient eligibility, the same risk factors and diagnostic criteria as in the previously described trial were applied. However, for patients with radiological criteria, only, confirmatory microbiology or pathology results needed to be obtained within 4 days of randomization (“up-grading”) to allow for continuation of study treatment. The primary endpoint was defined as favorable outcome, i.e. complete or partial response at the end of study treatment. Secondary endpoints included survival up to 12 weeks and the safety profiles of the treatment regimens.
Favorable response rates of 50 % and 46 % were reported from the 3 and 10 mg/kg group, respectively, revealing no significant difference. However, significantly higher rates of nephrotoxicity and hypokalemia were observed in the high-dose L-AmB group (Cornely et al. 2007a).
Based on these findings, L-AmB at a dosage of 3 mg/kg qd is commonly recommended as an alternative to voriconazole in the setting of targeted or pre-emptive treatment. Although preference in patients without prior azole therapy and lack of contraindications may be given to voriconazole as first-line treatment, L-AmB may be preferred in patients with prior azole exposition, e.g. those receiving posaconazole prophylaxis.
For both studies, analyses of outcome by presence of diagnostic criteria (radiology, microbiology, and pathology) revealed superior response rates for patients included into the studies on the basis of a halo or air-crescent sign, only (Cornely et al. 2007a; Herbrecht et al. 2002; Greene et al. 2007). In these patients, therapy of IA was probably initiated earlier than in patients who had undergone a thorough workup and presented with further diagnostic criteria at randomization. These findings underline the necessity of early treatment initiation. However, this interpretation should not discourage physicians from completion of further diagnostic procedures, even after initiation of antifungal treatment, because alternative diagnoses might otherwise be missed.
The role of echinocandins as first-line treatment agents for IA has not been satisfactorily assessed. A small number of uncontrolled studies evaluated the role of caspofungin and micafungin in neutropenic cancer patients and/or those undergoing allogeneic SCT. From these studies, success rates between 33 % and 44 % have been reported (Herbrecht et al. 2010; Viscoli et al. 2009; Denning et al. 2006). The large number of severely ill patients enrolled into these studies might be partly responsible for these comparatively unfavorable response rates. Of note, in a phase II dosage escalation study using caspofungin 70, 100, 150 and 200 mg as first-line treatment for IA, escalation was associated with an increase of response to treatment (Cornely et al. 2011). However, further studies will be necessary to consolidate this hypothesis.
Concerning the efficacy of combination antifungal therapy as first-line treatment, there are no results available from properly randomized, controlled clinical trials. However, observational studies have provided some evidence for the use of voriconazole plus caspofungin (Marr et al. 2004a) or L-AmB plus caspofungin as second-line treatment (Raad et al. 2008; Maertens et al. 2006; Caillot et al. 2007).
B. Invasive Candidiasis
Although the only reliable diagnosis of IC is currently based on positive results from blood cultures, the true diagnostic yield of this method remains unknown, as no other gold standard has been established so far. This unsatisfactory situation has led to the development of various surrogate markers and risk scores, intended for early identification of patients at risk of IC. Similar attempts have been made to identify patients who might profit from early initiation of antifungal prophylaxis for IC (see Sect. II); however, these scoring systems usually do not include any surrogate markers. In the following section, an overview on the most relevant concepts will be given (Table 14.3).
Table 14.2
Treatment of invasive candidiasis in ICU and neutropenic patients
Indication | Antifungal agent | Dosage | Reference |
|---|---|---|---|
Non-neutropenic patients | Anidulafungin | 200 mg qd loading dose and 100 mg qd maintenance iv | Reboli et al. (2007) |
Micafungin | 100 mg qd iv | ||
Caspofungin | 70 mg qd loading dose, 50 mg qd maintenance iv | ||
Neutropenic patients | Micafungin | 100 mg qd iv | |
Caspofungin | 70 mg qd loading dose and 50 mg qd maintenance iv | ||
Second-line neutropenic and non-neutropenic patients | L-AmB | 3 mg/kg qd iv | Kuse et al. (2007) |
Voriconazole | 6 mg/kg bid loading dose, followed by 4 mg/kg bid iv | Kullberg et al. (2005) |
In the 1990s, Pittet et al. identified the high relevance of colonization with Candida spp. prior to the development of candidemia on the basis of a prospective cohort study. To identify patients at risk, the Candida colonization index (CI) and the corrected Candida colonization index (CCI) were developed (Table 14.3) (Pittet et al. 1994). This work established colonization as a crucial risk factor to be incorporated in most successive scoring systems. For example, in a prospective study including surgical ICU patients, the CCI with a threshold of 0.4 was used to identify patients eligible for pre-emptive therapy with fluconazole. In this setting, the incidence of proven IC acquired in the ICU decreased from 2.2 % to 0 % (P < .001). This intervention did not lead to a detectable emergence of Candida strains resistant to fluconazole (Piarroux et al. 2004). Two further analyses in medical and surgical ICU patients, however, failed to identify an association between previous colonization with Candida spp. and patient outcome (Charles et al. 2005; Troughton et al. 2010). It was hypothesized that the identification of further independent risk factors for IC, besides colonization, might result in an improved predictive model.
Table 14.3
Candida spp. pre-emptive treatment scores
Reference | Study type | Inclusion criteria | Number of patients (n) | Best rule | Sensitivity | PPV |
|---|---|---|---|---|---|---|
(specifity) (%) | (NPV) (%) | |||||
Pittet et al. (1994) | Prospective cohort | Colonization with Candida spp., defined as detection in three or more samples taken from the same or different body sites on at least two consecutive screening days. | 650 screened, 29 included | CIa | 100 | 66 |
(69) | (100) | |||||
CCIb | 100 | 100 | ||||
(100) | (100) | |||||
Piarroux et al. (2004) | Prospective cohort | Surgical ICU ≥5 days | Prospective cohort: 899 screened, 413 included | CCIb ≥0.4 | NA | NA |
Retrospective cohort | Retrospective cohort: 906 screened, 455 included | |||||
Charles et al. (2005) | Prospective cohort | Medical ICU ≥7 days, | 593 screened, 92 included | CIa ≥0.5 | NA | NA |
Leon et al. (2006) | Prospective cohort | Medical and/or surgical ICU ≥7 days | 1,765 screened, 1,669 included | CSc ≥2.5 | 81 | NA |
(74) | ||||||
Leon et al. (2009) | Prospective cohort | Medical and/or surgical ICU ≥7 days | 1,205 screened, 1,107 included | CSc ≥3 | 78 | NA |
(66) | ||||||
Troughton et al. (2010) | Retrospective cohort | Surgical ICU | 3,500 screened, 974 included | Candida spp. colonization at two or more non-sterile sites and signs of unexplained sepsis | NA | NA |
Leon et al. (2012) | Prospective cohort | Medical and/or surgical ICU ≥7 days, severe abdominal condition | 338 screened, 176 included | BDG >259 pg/ml and positive CAGTA | 90 | 42 |
(55) | (94) |
Consequently, Leon et al. identified four independent risk factors for development of IC in a population of 1,669 non-neutropenic ICU patients. The statistical weight of each of these risk factors was used to create the Candida score. Patients with at least 2.5 points were found to be at a 7.75-fold risk of IC and therefore eligible for pre-emptive treatment (Leon et al. 2006). In a follow-up study by the same authors, the optimum cutoff was adjusted to 3 points (Leon et al. 2009). In a more recent study, the authors presented the combination of a β-d-glucan value of >259 pg/ml and a positive Candida albicans germ tube antibody test as a reliable predictor of IC (Leon et al. 2012). Table 14.3 provides an overview on studies analyzing scoring systems for pre-emptive treatment.
D-AmB used to be considered the gold standard in the treatment of candidemia until a series of clinical trials assessing the clinical efficacy of less toxic antifungals was initiated in the 1990s with a multicenter, randomized trial comparing the clinical efficacy of D-AmB and fluconazole in the treatment of IC in immunocompetent, non-neutropenic patients. With corresponding response rates of 79 (n = 81) and 70 % (n = 72), respectively, there was no significant difference in treatment outcome (P = 0.22). However, treatment with fluconazole was accompanied by less side effects than with D-AmB (Rex et al. 1994).
Because the proportion of fluconazole-resistant Candida non-albicans strains (e.g. Candida krusei or Candida glabrata) has been continuously growing, other antifungals with a broader spectrum than fluconazole have quickly become the focus of further studies. A multicenter, randomized, non-inferiority study compared intravenous voriconazole to D-AmB followed by oral or intravenous fluconazole in non-neutropenic patients with candidemia. The primary endpoint, defined as sustained clinical and mycological response, 12 weeks after end of treatment, was reached by 41 % of patients in each group. In the voriconazole group, fewer serious adverse events, especially those classified as renal toxicity were observed (Kullberg et al. 2005).
Similarly favorable results were expected from the newly developed echinocandins. Caspofungin was compared to D-AmB in the treatment of IC in a mixed population of non-neutropenic (n = 200) and neutropenic patients (n = 24). With 73.4 % and 61.7 %, respectively, response rates between caspofugin and D-AmB did not differ significantly. Patients in the D-AmB group, however, experienced more nephrotoxic effects and hypokalemia (Mora-Duarte et al. 2002). In a double-blind, randomized, multinational non-inferiority trial, first-line treatment with micafungin and L-AmB was compared in non-neutropenic (n = 469) and neutropenic (n = 62) patients. Non-inferiority criteria were met, and micafungin was associated with fewer treatment-related adverse events than L-AmB (Kuse et al. 2007). Another randomized, double-blind trial compared micafungin 100 mg qd (n = 191) to micafungin 150 mg qd (n = 199) and the standard dosage of caspofungin (n = 188) in patients with invasive candidiasis. There was no significant difference in the response rate between the three treatment arms. Therefore, dosages of micafungin 100 mg qd and 150 mg qd were regarded as non-inferior to a standard dosage of caspofungin (Pappas et al. 2007).
Finally, a randomized, double-blind, multicenter trial comparing anidulafungin with fluconazole for the treatment of IC was conducted in a predominantly non-neutropenic population. Significantly higher response rates (75.6 % versus 60.2 %) and better survival (74 % versus 69 %) were documented for patients treated with anidulafungin (P = 0.01) (Reboli et al. 2007). Results from clinical trials on the treatment of IC are summarized in Table 14.2.
C. Emerging Fungi
As the range of emerging fungi is diverse, only the clinically most important ones will be covered in this section, including Mucorales, Fusarium spp., Scedosporium spp., and rare yeasts. There is very limited data from controlled randomized clinical trials available for any of the emerging fungi. Available data remains mostly restricted to microbiological studies, case series, and animal studies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree