Despite the use of traditional antianginal medications (i.e., β blockers, calcium channel blockers, and nitrates) and revascularization therapies, symptoms of chronic stable angina pectoris (CSAP) persist in ≥25% of patients. The objective of this systematic review was to synthesize the available evidence from randomized controlled trials (RCTs) of ranolazine for the treatment of CSAP. We systematically searched the Cochrane Register of Controlled Trials, EMBASE, and MEDLINE through July 2013 for RCTs comparing ranolazine with placebo or antianginal medications administered as part of usual care for the management of CSAP. End points of interest included exercise stress test performance (duration, time to angina, and time to ST-segment depression), frequency of angina attacks/week, nitroglycerin use/week, and quality of life. We identified 7 RCTs (n = 3,317) of patients with CSAP due to coronary artery disease. Comparators included placebo, amlodipine, and atenolol. All but 1 trial showed a statistically significant improvement in all 3 exercise stress test parameters with ranolazine compared with placebo. Ranolazine also reduced angina frequency and nitroglycerin use compared with placebo. These findings were consistent whether or not patients were also prescribed traditional antianginal pharmacotherapy. In conclusion, ranolazine reduces anginal symptoms among patients with symptomatic CSAP despite their use of traditional antianginal medications.
Affecting >7 million North Americans, chronic stable angina pectoris (CSAP) has a major negative impact on general health status and quality of life. Despite the use of antianginal medications and revascularization therapies, previous studies have estimated that ≥25% of patients remain symptomatic 1 year after undergoing coronary artery bypass grafting or percutaneous coronary intervention. Traditional antianginal drugs such as β blockers, calcium channel inhibitors, and long-acting nitrates can result in low blood pressure and heart rate. However, ranolazine, a piperazine derivative, produces its antianginal effects without decreasing heart rate or blood pressure. Our objective was to assess the efficacy and safety of ranolazine through a systematic review of randomized controlled trials (RCTs) comparing ranolazine with placebo or pharmacotherapies used as part of usual care for the treatment of CSAP.
Methods
We performed this systematic review following a prespecified protocol and reported it following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. We systematically searched the Cochrane Register of Controlled Trials, EMBASE, and MEDLINE from inception to July 2013 using the search term ranolazine. The search was restricted to RCTs using a modified version of the McMaster RCT hedge. Our search was further limited to studies conducted in humans and published in English or French.
Included studies were (1) conducted in patients with CSAP due to coronary artery disease (CAD), (2) RCTs comparing ranolazine with placebo or conventional treatment, (3) double-blind trials, including crossover studies, (4) published in English or French, (5) conducted in adults aged ≥18 years, and (6) studies with treatment duration ≥7 days. We excluded open-label trials because of the subjective nature of the outcomes assessed, as well as observational studies, case series, and case reports. In addition, we excluded letters to the editor, editorials, reviews, and abstracts from conference proceedings. Patients were excluded if they had factors that limit electrocardiographic interpretation (e.g., pacemaker, left bundle branch block, digoxin), significant hepatic and/or renal impairment, acute coronary syndrome or revascularization in the previous 2 months, New York Heart Association III or IV heart failure, or QTc ≥500 ms.
Two reviewers performed data extraction, with disagreements resolved by consensus or a third reviewer. Extracted data included patient and study characteristics, as well as safety outcomes. Baseline participant characteristics included age, gender, and presence of symptomatic chronic angina (≥3 months). Data related to the formulation of ranolazine (immediate release vs extended release) and drug discontinuation were also extracted. Outcomes of interest included exercise stress test (EST) duration, time to onset of angina, time to onset of ST-segment depression, amount of nitroglycerin used per week, frequency of angina attacks per week, and quality of life using the Seattle Angina Questionnaire (SAQ). Safety end points were minor/major adverse events and long-term mortality (≥1 year).
Two reviewers independently evaluated the quality of included RCTs using the Cochrane Collaboration tool for assessing risk of bias. This tool evaluates the risk of bias in 6 different domains: selection, performance, detection, attrition, reporting, and other. For each included RCT, we assessed the potential presence of these biases and assigned a “low,” “high,” or “unclear” risk to each domain.
Results
Our systematic search yielded 339 potentially relevant reports. Following title and abstract screening, we retrieved the full-length texts of 12 potentially relevant publications and evaluated them for eligibility. Of these, 7 studies met our inclusion criteria and were included in our systematic review ( Figure 1 ). The Monotherapy Assessment of Ranolazine In Stable Angina (MARISA) trial and the trial by Thadani et al compared ranolazine with placebo on no background medication (n = 510). The Combination Assessment of Ranolazine In Stable Angina (CARISA) and Type 2 Diabetes Evaluation of Ranolazine in Subjects With Chronic Stable Angina (TERISA) trials and the trial by Pepine and Wolff compared ranolazine with placebo on some background antianginal medications (n = 2,084). The Efficacy of Ranolazine In Chronic Angina (ERICA) trial compared ranolazine with placebo on background maximal dose of amlodipine (n = 565), and the Ranolazine Versus Atenolol for Chronic Angina Pectoris (RAN080) trial, a 3-armed RCT, compared ranolazine with atenolol and placebo (n = 158). Overall, included studies had a low risk of bias ( Figure 2 ).
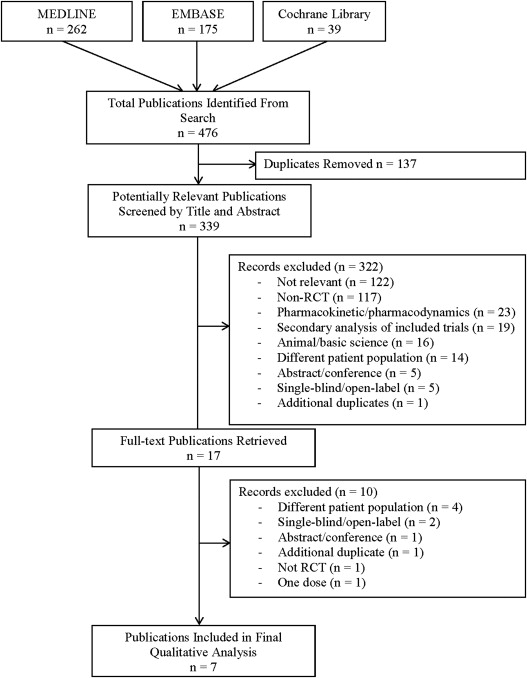
A total of 3,301 patients were included in the 7 RCTs ( Table 1 ). Patients were predominantly men, had CSAP (≥3-month duration) with evidence of CAD (previous myocardial infarction, stenosis present on angiogram, or evidence of myocardial perfusion defects on noninvasive stress imaging), and baseline EST duration in the range of 3 to 13 minutes (i.e., moderate to severe). The median treatment duration ranged from 7 to 84 days, and the maximum follow-up was 730 days. Efficacy outcomes varied among studies. Most studies included safety outcomes of interest (i.e., mortality, major/minor symptoms, duration of QT). Ranolazine dosages ranged from 30 mg 3 times/day (TID) to 1,500 mg twice/day (BID). Three studies used the immediate-release formulation, with the remaining studies using the sustained-release formulation of the drug.
| Trial | Trial Design | Participants | Key Inclusion Criteria ∗ | Medication Protocol | Dose and Treatment Duration | Outcome | |
|---|---|---|---|---|---|---|---|
| Active | Control | ||||||
| TERISA (2013) | Parallel | 462 | 465 | T2DM, CAD, CSAP ≥3 months, max 2 AA drugs | If >2 AA drugs at screening, Tx W/O >2 weeks of placebo run-in | 500 and 1,000 mg BID † (3 weeks) | Angina freq. |
| CARISA (2004) | Parallel | 554 | 269 | CAD, CSAP ≥3 months, ST-D ≥1 mm, EST 3–9 minutes | Non-background AA Tx D/C ≥5 days before qualifying EST | 750 and 1,000 mg BID ‡ (12 weeks) | EST duration, time to angina, time to ST-D, angina freq., NG use |
| ERICA (2006) | Parallel | 281 § | 284 § | CAD, CSAP ≥3 months | No AA Tx except LAN and NG, amlodipine continued | 1,000 mg BID ‡ (6 weeks) | Angina freq., NG use, SAQ |
| Thadani et al (1994) | Parallel | 240 | 79 | CSAP ≥3 months, ST-D ≥1 mm, EST 3–9 minutes | 5-day W/O, 7–12 day placebo run-in, 4-week double-blind phase | 30, 60, and 120 mg TID (4 weeks) | Change from baseline EST, angina freq. |
| Pepine and Wolff (1999) | 5 X-over | 318 | CSAP ≥3 months, ST-D ≥1 mm | All other AA drugs kept constant | 400 mg BID, 267 mg TID, 400 mg TID ‖ (5 weeks) | EST duration, time to angina, time to ST-D | |
| MARISA (2004) | 4 X-over | 191 | CAD, CSAP ≥3 months | D/C all AA Tx (except NG) | 500, 1,000, and 1,500 mg BID ‡ (4 weeks) | EST duration, time to angina, time to ST-D | |
| RAN080 (2005) | 3 X-over | 158 ¶ | CAD, CSAP, ST-D ≥1 mm, EST 3–9 minutes | SAN, CCB constant. Other AA Tx D/C before placebo W/O | 400 mg TID RAN vs ATE ‖ (3–4 weeks) | EST duration, time to angina, time to ST-D | |
∗ Trials reported heterogeneous inclusion criteria, including differences in reported angina frequency and NG use, background treatment, placebo run-in phase, and change in qualifying EST performance. Key clinical criteria have been included in the table.
† If 500 mg was well tolerated for 1 week, dosage was increased to 1,000 mg BID (patients on verapamil or diltiazem remained on 500 mg ranolazine or placebo).
§ A total of 565 patients (281 active and 284 control) were randomized, of whom 558 (277 active and 281 control) were included in angina and NG frequency analyses.
¶ A total of 158 patients were randomized, of whom 154 had outcome data for EST duration, mean increase in time to angina, and time to ST-D.
Baseline patient characteristics were well balanced across treatment groups, including background antianginal medications, angina duration, baseline frequency of angina attacks, and nitroglycerin use ( Table 2 ). The CARISA trial compared placebo with ranolazine 750 mg BID versus 1,000 mg BID, with EST improvement as an end point. Each treatment arm had similar background antianginal medications (43% atenolol, 31% amlodipine, and 26% diltiazem). In the ERICA trial, both the placebo and treatment groups were on similar background amounts of long-acting nitrates (43% vs 46%, respectively), and all patients were taking amlodipine 10 mg/day. In the trial by Thadani et al and CARISA trial, the ranolazine group had a greater prevalence of previous coronary artery bypass graft surgery and less prevalence of heart failure relative to placebo. However, statistical adjustments were used to account for those statistical differences in the efficacy analyses.
| Trial | Background Drugs, % | Men (%) | Age (yrs) | Angina Duration (yrs) | Angina/Week (Mean) | Nitroglycerin/Week (Mean) | ||
|---|---|---|---|---|---|---|---|---|
| BB | CCB | LAN | ||||||
| TERISA (2013) | 90.2 | 28.8 | 33.7 | 61.4 | 63.7 | — | — | — |
| CARISA (2004) | 43.1 | 26, 31 ∗ | 0 | 77.5 | 63.9 | — | 4.5 | 3.9 |
| ERICA (2006) | 0 | 100.0 | 44.5 | 72.5 | 61.7 | — | 5.6 | 4.7 |
| Thadani et al (1994) | 0 | 0 | 0 | 79.0 | 65.3 | 5.4 | 6.0 | — |
| Pepine and Wolff (1999) | 34.0 | 24.0 | 0 | 72.0 | 64.2 | 5.8 | 2.3 | — |
| MARISA (2004) | 0 | 0 | 0 | 73.3 | 64.3 | — | — | — |
| RAN080 (2005) | 0 | 54.0 | 11.0 | 89.0 | 59.0 | — | — | — |
∗ CARISA trial reported 2 calcium channel blockers: 26% of participants were taking diltiazem and 31% of participants were taking amlodipine.
A total of 5 included RCTs examined change in EST performance ( Tables 3 and 4 ). In terms of total EST duration, the trial by Thadani et al showed no difference between ranolazine and placebo at both peak and trough times. In the trial by Pepine and Wolff, the ranolazine group had a significant increase of 0.20 minutes (267 mg TID) over the placebo group at peak time (1 hour) but similar improvement compared with placebo at trough time. Three trials reported improvements in total EST duration. The MARISA and CARISA trials showed increases of 0.93 minutes (1,500 mg BID) and 0.55 minutes (750 mg BID) over the placebo group at peak time and of 0.77 minutes (1,500 mg BID) and 0.36 minutes (750 mg BID) at trough time (12 hours), respectively. Finally, the RAN080 trial showed an increase of 0.62 minutes (400 mg TID) relative to placebo and 0.35 minutes (400 mg TID) over atenolol at a peak time of 1 hour (trough time was not assessed).
| Trial | Tx Groups | EST Duration ∗ | Time to Angina ∗ | Time to ST-D ∗ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Absolute Post-Tx † | Change from Baseline | Baseline | Absolute Post-Tx † | Change from Baseline | Baseline | Absolute Post-Tx † | Change from Baseline | ||
| CARISA (2004) | Placebo | 7.78 (0.14) | 8.87 | 1.09 (0.14) | 6.49 (0.14) | 7.97 | 1.48 (0.16) | 6.74 (0.16) | 7.73 | 0.99 (0.15) |
| 750 mg BID | 7.76 (0.14) | 9.42 | 1.66 (0.13) | 6.46 (0.14) | 8.58 | 2.12 (0.15) | 6.84 (0.16) | 8.51 | 1.67 (0.15) | |
| 1,000 mg BID | 7.84 (0.13) | 9.37 | 1.53 (0.13) | 6.39 (0.14) | 8.50 | 2.11 (0.16) | 6.67 (0.17) | 8.23 | 1.56 (0.15) | |
| Thadani et al (1994) | Placebo | 5.93 (0.24) | 6.41 | 0.48 (0.15) | 4.52 (0.26) | 5.03 | 0.51 (0.19) | 4.30 (0.28) | 4.71 | 0.41 (0.21) |
| 30 mg TID | 6.41 (0.25) | 6.73 | 0.32 (0.15) | 5.16 (0.27) | 5.75 | 0.59 (0.19) | 4.53 (0.28) | 4.97 | 0.44 (0.21) | |
| 60 mg TID | 5.86 (0.25) | 6.48 | 0.62 (0.15) | 4.47 (0.27) | 5.37 | 0.90 (0.19) | 4.02 (0.28) | 4.78 | 0.76 (0.21) | |
| 120 mg TID | 6.60 (0.25) | 7.05 | 0.45 (0.15) | 4.97 (0.26) | 5.63 | 0.66 (0.19) | 4.85 (0.28) | 5.35 | 0.50 (0.21) | |
| Pepine and Wolff (1999) | Placebo | — | 10.67 | — | — | 9.01 | — | — | 9.58 | — |
| 267 mg TID | — | 10.87 | — | — | 9.40 | — | — | 9.99 | — | |
| 400 mg BID | — | 10.83 | — | — | 9.33 | — | — | 9.86 | — | |
| 400 mg TID | — | 10.84 | — | — | 9.33 | — | — | 9.94 | — | |
| MARISA (2004) | Placebo | — | 8.36 (0.09) | — | — | 6.94 (0.10) | — | — | 7.27 (0.14) | — |
| 500 mg BID | — | 8.85 ‡ | — | — | 7.53 ‡ | — | — | 7.92 ‡ | — | |
| 1,000 mg BID | — | 9.20 ‡ | — | — | 7.88 ‡ | — | — | 8.20 ‡ | — | |
| 1,500 mg BID | — | 9.29 ‡ | — | — | 8.08 ‡ | — | — | 8.42 ‡ | — | |
| RAN080 (2005) | 400 mg TID vs placebo | — | 0.62 § | — | — | 0.85 § | — | — | 0.88 § | — |
| 400 mg TID vs atenolol | — | 0.35 § | — | — | 0.19 § | — | — | 0.03 § | — | |
∗ All times are presented as mean (standard error) and reported in minutes.
† Absolute post-treatment differences calculated manually.
‡ MARISA reported values for placebo and difference from placebo. Difference was added to placebo value to obtain absolute post-treatment values.
§ RAN080 reported absolute difference in change between treatment group and placebo.
| Trial | Tx Groups | EST Duration ∗ | Time to Angina ∗ | Time to ST-D ∗ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Absolute Post-Tx † | Change from Baseline | Baseline | Absolute Post-Tx † | Change from Baseline | Baseline | Absolute Post-Tx † | Change from Baseline | ||
| CARISA (2004) | Placebo | 6.97 (0.11) | 8.50 | 1.53 (0.14) | 5.45 (0.11) | 7.36 | 1.91 (0.15) | 4.98 (0.15) | 7.07 | 2.09 (0.15) |
| 750 mg BID | 6.94 (0.10) | 8.86 | 1.92 (0.13) | 5.41 (0.11) | 7.81 | 2.40 (0.15) | 5.17 (0.15) | 7.59 | 2.42 (0.15) | |
| 1,000 mg BID | 6.91 (0.11) | 8.84 | 1.93 (0.14) | 5.45 (0.11) | 7.79 | 2.34 (0.15) | 5.03 (0.15) | 7.47 | 2.44 (0.16) | |
| Thadani et al (1994) | Placebo | 5.93 (0.24) | 6.51 | 0.58 (0.17) | 5.52 (0.26) | 6.23 | 0.71 (0.20) | 4.30 (0.28) | 4.62 | 0.32 (0.22) |
| 30 mg TID | 6.41 (0.24) | 6.76 | 0.35 (0.17) | 5.16 (0.26) | 5.87 | 0.71 (0.20) | 4.53 (0.28) | 5.01 | 0.48 (0.22) | |
| 60 mg TID | 5.97 (0.25) | 6.49 | 0.52 (0.17) | 4.52 (0.27) | 5.38 | 0.86 (0.20) | 4.14 (0.28) | 4.90 | 0.76 (0.22) | |
| 120 mg TID | 6.60 (0.24) | 7.05 | 0.45 (0.17) | 4.97 (0.26) | 5.48 | 0.51 (0.20) | 4.85 (0.27) | 5.43 | 0.58 (0.21) | |
| Pepine and Wolff (1999) | Placebo | — | 10.50 | — | — | 8.58 | — | — | 9.04 | — |
| 267 mg TID | — | 10.56 | — | — | 8.78 | — | — | 9.22 | — | |
| 400 mg BID | — | 10.55 | — | — | 8.77 | — | — | 9.22 | — | |
| 400 mg TID | — | 10.60 | — | — | 8.65 | — | — | 9.31 | — | |
| MARISA (2004) | Placebo | — | 8.43 (0.10) | — | — | 6.79 (0.11) | — | — | 7.39 (0.10) | — |
| 500 mg BID | — | 8.83 ‡ | — | — | 7.38 ‡ | — | — | 7.85 ‡ | — | |
| 1,000 mg BID | — | 8.99 ‡ | — | — | 7.56 ‡ | — | — | 8.13 ‡ | — | |
| 1,500 mg BID | — | 9.20 ‡ | — | — | 7.78 ‡ | — | — | 8.47 ‡ | — | |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


