The Thymus
Thomas W. Shields
Embryology
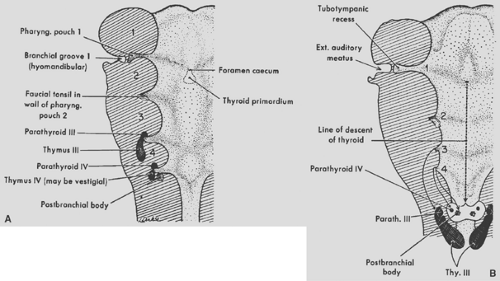
The thymus in humans primarily arises from the third pharyngeal pouches (thymus III). Thymic tissue may also arise from the fourth pharyngeal pouches (thymus IV), but in humans, these latter primordia may be altogether absent or rudimentary and give rise only to vestigial tissue masses. Patton41 noted that thymic IV tissue, when present, usually becomes associated with the thyroid and may ultimately become embedded within its substance.
According to Patton,41 the thymic primordia appear late in the sixth week of gestation as ventral outgrowths of the third pharyngeal pouches. It is believed that both the ventral pharyngeal pouch III endoderm and the ectoderm of the floor of the branchial furrow from fusion of the second, third, and fourth branchial clefts contribute to the primordial cell mass. Norris39 and others postulated that some of the epithelial components of the thymus arise from the ectodermal cells of the aforementioned branchial clefts. von Gaudecker’s52 studies support this concept, and he believes that the ectodermal cells from the cervical vesicle primarily of the third cleft actively proliferate, migrate, and completely surround the endodermal thymic primordium from the third pharyngeal pouch. Ectodermal cells are thus thought to be mainly the origin of the epithelial cells of the cortex and endodermal cells the source of the medullary epithelial cells, but this is far from settled. Kornstein,26 Norris,39 and Weller54 each studied the same collection of embryos and reached opposite conclusions. Also, immunohistochemical studies have shown that human thymic epithelial cells at 7 weeks’ development all express common antigens. Thus, Kornstein26 notes that it is possible that thymic epithelial cells derive from a common stem cell. Divergence occurs later in differentiation of epithelial subtypes, as suggested by the immunohistochemical differences in newborn and adult thymic cells in the subcapsular, cortical, and medullary areas.
Morphologic and immunohistochemical studies done by McFarland,35 Haynes,19 De Maagd,12 and von Gaudecker53 and their associates have demonstrated that detectable immunohistochemical differences (expression of distinctive surface antigens) exist between the epithelial cells of the thymic cortex and the thymic medulla. Hirokawa and colleagues21 also reported that the localization of various thymic epithelial cell markers in the newborn human thymus reflects possible different phenotypic origins of epithelial cells in the cortex and medulla (Table 163-1). Marino and Müller-Hermelink33 and Müller-Hermelink and colleagues36 used these immunohistochemical and morphologic differences of the cortical and medullary epithelial cells to classify epithelial thymic tumors (thymomas) (see Chapter 188). Hirokawa and associates21 confirmed these differences and noted that most thymic tumors (thymomas) are of cortical epithelial cell origin.
The primordia of the thymus elongate rapidly in the seventh week but retain their connection with the third pouch and remain associated with parathyroid tissue III (Fig. 163-1). The right and left thymic tissue masses move toward the midline just caudal to the thyroid primordium and by the eighth week make contact with each other but do not fuse. They remain separate but connected lobes. The gland migrates caudad and slides under the sternum in front of the great vessels into the mediastinum to lie in contact with the superior portion of the ventral aspect of the pericardium (Fig. 163-2).
Gross Anatomy
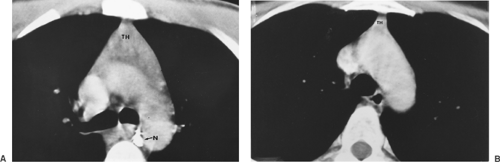
In the newborn, according to the studies of Boyd8 and Kendall and associates,25 the gland reaches a mean weight of 15 g in the early neonatal period. Steinmann,47 however, reported that the weight in grams in children from newborn to the age of 1 was 27.3 g, with a standard deviation of 16.4 g. The gland reaches its largest relative size in childhood, although it continues to grow until puberty to a mean weight of 30 to 40 g. The recorded weight is less in Steinmann’s47 studies. A gradual process of involution then occurs throughout adulthood, and the gland is reduced in weight to between 5 and 25 g. A study by Steinmann,47 as noted, found the mean weight of the thymus in the various age groups to be generally less than the aforementioned mean weights. In all the age groups except for individuals >85 years of age, the mean thymic weight varied between 20 and 28 g, with an average standard deviation of 12.5. In very old individuals, mean weight varies from 18 g in individuals aged 85 to 90 years to 12 g in those 91 to 107 years of age, with a mean standard deviation of 5.4 and 6.9, respectively. Figure 163-3 shows the variations in size of the gland on computed tomography (CT) in an adolescent boy and an adult woman.
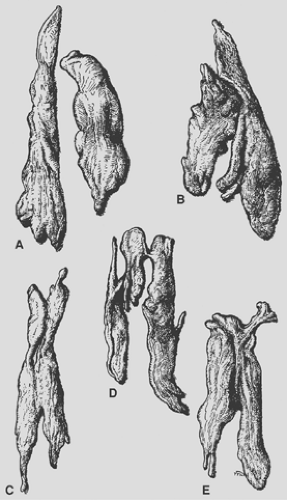
The thymus, although classically described as having a right and left lobe, may actually be a composite of three or more lobular structures. Nonetheless, the gland tends to maintain its original paired character. The lobules are generally asymmetric (Fig. 163-4). Grossly, the gland has a roughly H-shaped
configuration, with extension of the upper poles of either side into the base of the neck, and with a greater or lesser attachment to the thyroid gland by the thyrothymic ligament. The lower poles of each side extend down over the pericardium.
configuration, with extension of the upper poles of either side into the base of the neck, and with a greater or lesser attachment to the thyroid gland by the thyrothymic ligament. The lower poles of each side extend down over the pericardium.
Table 163-1 Localization of Various Markers in Epithelial Cells of Newborn Human Thymus | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||
In addition to the variations in size and configuration of the thymic gland, numerous collections of varying amounts of identifiable thymic tissue, both gross and microscopic, may be found as additional mediastinal lobes and islets of tissue outside the capsule of the “gland” extending from the neck to the diaphragm. In a small series of patients, Masaoka and colleagues34 identified a 72% incidence of microscopic collections of thymic tissue in the mediastinal fatty tissues of the anterior compartment outside the thymic capsule. Fukai and colleagues14 described a 51.8% incidence of this phenomenon in a series of
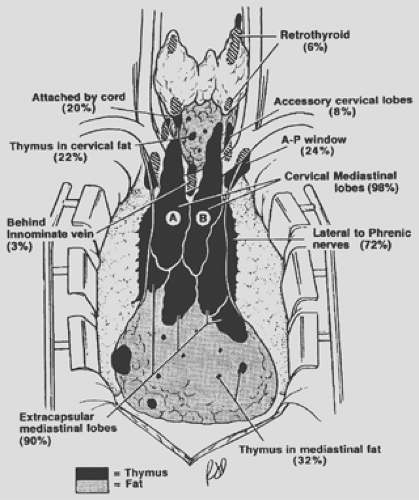
27 subjects. Thymic tissue was found in anterior mediastinal fat in 44.4% of specimens and in 7.4% of retrocarinal fat; none was found in preaortic fatty tissues. Ashour,3 in surgical dissection in 38 myasthenic patients, reported an incidence of 39.5% of ectopic thymic tissue foci. Nineteen sites were located in 15 patients: 12 (63%) were in the neck, and 4 patients (21%) had thymic tissue in fat of the cardiophrenic angle. The variations in location of thymic tissue outside the gland were also well documented by the studies of Jaretzki and Wolff23 and Jaretzki.22 The cervical and mediastinal variations in the location of these thymic tissue foci are schematically illustrated in Figure 163-5.
27 subjects. Thymic tissue was found in anterior mediastinal fat in 44.4% of specimens and in 7.4% of retrocarinal fat; none was found in preaortic fatty tissues. Ashour,3 in surgical dissection in 38 myasthenic patients, reported an incidence of 39.5% of ectopic thymic tissue foci. Nineteen sites were located in 15 patients: 12 (63%) were in the neck, and 4 patients (21%) had thymic tissue in fat of the cardiophrenic angle. The variations in location of thymic tissue outside the gland were also well documented by the studies of Jaretzki and Wolff23 and Jaretzki.22 The cervical and mediastinal variations in the location of these thymic tissue foci are schematically illustrated in Figure 163-5.
Thymic tissue may be found in the neck in about 36% of people, outside the “normal” cervical extensions of the thymic lobes. Most or all such tissue likely represents arrested descent of thymus III tissues. In the mediastinum, almost all individuals have some thymic tissue beyond the confines of the classic mediastinal lobes. Unencapsulated thymic tissue can be found in the region of the phrenic nerves, behind the innominate vein, in the aortopulmonary window, in the aortocaval groove, and in the anterior and cardiophrenic fatty tissues. The presence of thymic tissue in these locations readily explains the occasional occurrence of epithelial thymic tumors (see Chapter 188) and true thymic cysts (see Chapter 204) in these areas. The significance of these variations in location in the management of myasthenia gravis is discussed in Chapters 185 and 186.
In the adult, the upper portions of the gland normally lie on the anterior surface of the left innominate vein. On occasion, one or both lobes lie behind this vein instead of in front of it. Jaretzki and Wolff23 found both lobes posterior to the vein in 1 of 50 patients (2%) and one lobe in this position in 2 of 50 patients (4%). Other anomalies in position include partial or complete failure of descent of one or both thymic lobes and the presence of thymic tissue at the root of either lung or even within the pulmonary parenchyma. The inferior limits of the adult thymus in relation to the sternocostal levels have been recorded by Bell and colleagues6 (Fig. 163-6), and as noted by Jaretzki and Wolff,23 thymic tissue may be located as low as the cardiophrenic fat pads.
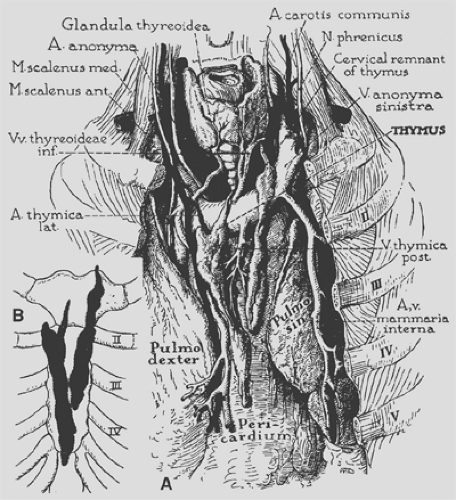
The arterial blood supply of the thymus (see Fig. 163-2) is mainly from branches of the internal mammary arteries, but the
gland may also receive small branches from the inferior thyroid arteries and the pericardiophrenic arteries. Venous drainage may be partially through small veins accompanying these arterial branches. The main drainage, however, is through a centrally located venous trunk on the posterior aspect of the gland that drains into the anterior aspect of the left innominate vein as a single vessel; occasionally a branch may enter the superior vena cava.
gland may also receive small branches from the inferior thyroid arteries and the pericardiophrenic arteries. Venous drainage may be partially through small veins accompanying these arterial branches. The main drainage, however, is through a centrally located venous trunk on the posterior aspect of the gland that drains into the anterior aspect of the left innominate vein as a single vessel; occasionally a branch may enter the superior vena cava.
No afferent lymph channels enter the gland, although efferent channels have been identified. These are believed to drain only the capsule and fibrous septa of the gland. These efferent channels terminate at the anterior mediastinal, pulmonary hilar, and internal mammary lymph nodes. Both sympathetic and parasympathetic nerve fibers enter the gland.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree