The Thoracic Duct and the Management of Chylothorax
Bradley M. Rodgers
Maxim Itkin
John C. Kucharczuk
Chylothorax is the abnormal accumulation of fluid from the lymphatic system within the pleural space. Chyle is not only composed mainly of the lymphatic drainage from the intestine but also includes lymph from the lungs, liver, abdominal wall, and the extremities. The amount of lymph originating from the extremities as a component of chyle is negligible under normal circumstances. Chylothorax may be either congenital or acquired (Table 27.1). Congenital chylothorax is thought to occur either secondary to disruption of the thoracic duct during delivery or to congenital anatomic abnormalities of the duct, such as atresias. Acquired chylothorax can arise from multiple causes. Disruption of the thoracic duct may occur due to blunt thoracic or cervical trauma. Occasionally, the duct is injured in penetrating thoracic trauma, although the posterior location of the duct makes this form of injury quite uncommon. The thoracic duct may be lacerated during placement of left subclavian venous catheter, however, far more commonly is injury to the duct or its tributaries, occurring during thoracic operations. Chylothorax has been estimated to occur after 0.25% to 0.50% of all intrathoracic operations and has been described after nearly every type of thoracic surgical procedure but appears to be more common following cardiac procedures requiring considerable mediastinal dissection at the base of the heart or after esophagectomy.
Mediastinal neoplasms are responsible for the majority of chylothoraces that develop spontaneously in adult patients. Most of these are secondary to obstruction of lymphatic pathways from mediastinal lymphomas. Infections are an uncommon cause of chylothorax in the United States, but tuberculous lymphadenitis is a more common cause in many other countries. Miscellaneous causes for chylothorax include thrombosis of the subclavian vein or superior vena cava (SVC), usually secondary to long-dwelling intravenous catheters, child abuse, and pulmonary lymphangiomatosis (Table 27.1).
 ANATOMY
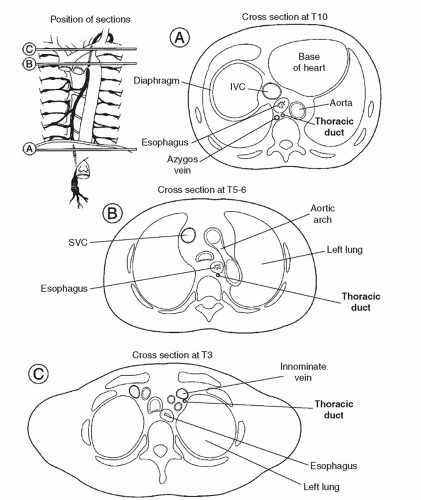
ANATOMYThe thoracic duct is the largest lymphatic channel in the body. It conveys the majority of the lymph within the body into the circulatory system. The duct arises embryologically as paired channels with numerous crossing anastomoses. In most instances, the paired structures fuse to form a singular vascular channel with the right duct persisting in the lower thorax and the left persisting in the upper chest (Fig. 27.1). Although up to 50% of individuals may have anomalous patterns of thoracic duct anatomy, in its most classic anatomic position the duct arises from the cisterna chyli anterior to the first or second lumbar vertebral body. The duct runs cephalad from the cisterna along the right side of the aorta and enters the chest through the aortic hiatus. The duct ascends in the right thorax, medial to the azygos vein and posterior to the esophagus. At the level of the fourth or fifth thoracic vertebra, the duct crosses anterior to the vertebral body and behind the esophagus to become a left-sided structure, dorsal to the aortic arch. The duct passes through the thoracic inlet posterior and to the left of the esophagus and forms an arch that rises 3 to 4 cm above the clavicle to the level of the sixth or seventh cervical vertebrae. It crosses anterior to the subclavian artery and the thyrocervical trunk as it extends laterally and terminates by opening into the angle of the junction of the left subclavian and the internal jugular veins (Fig. 27.2). A bicuspid valve at the lymphaticovenous junction prevents the reflux of blood into the duct at this junction. The duct is 3 to 5 mm in diameter at its origin in an adult, but its caliber diminishes in the mid-thorax to dilate again just proximal to its venous termination. The duct receives numerous lymphatic tributaries from the thoracic wall as it ascends through the chest, and there are multiple lymphaticovenous anastomoses between the duct and the azygos and intercostal veins. Riquet described two major thoracic duct tributaries from the heart. The right efferent trunk primarily drains lymph from the right ventricle and ascends between the aorta and pulmonary artery, connecting to the thoracic duct high in the left chest. The left efferent trunk drains lymph primarily from the left ventricle and ascends behind the pulmonary artery, usually connecting with the azygos vein in the right chest. Injury to the right efferent trunk may account for many cases of chylothorax or chylopericardium after cardiac surgery. Although this represents the most common anatomic configuration of the duct, there are numerous variations that may be of considerable surgical significance. The most common variation in anatomy is a duplicate duct, occasionally at the lower thoracic, but more commonly at the cervical level. The level at which the duct crosses the vertebral column may also be variable.
PHYSIOLOGY
There are multiple valves throughout the length of the thoracic duct, particularly in the cephalic end, that ensure unidirectional flow. The wall of the thoracic duct contains smooth muscle cells with an intrinsic contraction interval of 10 to 15 seconds. Movement of chyle through the thoracic duct is modulated primarily by the intrinsic contraction of the duct wall and the pressure gradient between the abdomen and thorax. The rate of lymph formation from the intestine and liver also affects the rate of flow through the duct. Flow through the thoracic duct varies between 0.38 and 3.9 ml/min.
The function of the thoracic duct is to transport ingested fat and lymphatic fluid from the abdominal viscera and lower body into the venous circulation. Approximately 60% to 70% of all ingested fat is absorbed by the intestinal lymphatics and transported by the thoracic duct. Fatty acids containing <10 carbon atoms are absorbed directly into the portal venous system, whereas
larger fats are formed into chylomicrons and transported into the lymphatics. The thoracic duct is also the main pathway for return of extravascular plasma proteins and lymphocytes to the vascular space. Prolonged loss of thoracic duct lymph can lead to fat and protein malnutrition as well as to immunocompromise secondary to loss of T lymphocytes.
larger fats are formed into chylomicrons and transported into the lymphatics. The thoracic duct is also the main pathway for return of extravascular plasma proteins and lymphocytes to the vascular space. Prolonged loss of thoracic duct lymph can lead to fat and protein malnutrition as well as to immunocompromise secondary to loss of T lymphocytes.
Table 27.1 Etiology of Chylothorax | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||
 DIAGNOSIS
DIAGNOSISThe diagnosis of chylothorax is suspected with the development of a pleural effusion in certain high-risk clinical settings, such as after esophagectomy. The diagnosis is confirmed by examination of the pleural fluid obtained by thoracentesis. In an individual consuming a normal diet, the diagnosis is usually quite evident, with a milky appearance to the fluid. Often, however, the patient has not received normal fats enterally before the development of the chylothorax, and the fluid in this clinical setting has the appearance of serum. Chemical analysis of the fluid reveals elevated triglyceride and total protein levels (Table 27.2). Cell counts reveal a marked predominance of lymphocytes, with numbers ranging from 400 to 7000/ml. Chronic pleural effusions secondary to tumors or infections may occasionally appear milky because of the accumulation of cholesterol in the fluid. This so-called pseudochylothorax can be differentiated from true chylothorax by the determination of the triglyceride level in the effusion. Most chylous effusions have a cholesterol/triglyceride ratio of <1, whereas nonchylous effusions have a ratio of >1. Pleural fluid with a triglyceride level of >110 mg/dl has a 99% chance of being chyle. If the triglyceride level is <50 mg/dl, the probability of a chylous effusion is only 5%.
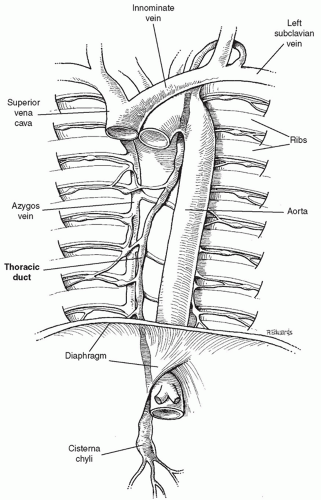 Fig. 27.1. The most common anatomic pattern of the thoracic duct. The duct enters the chest as a right-sided structure and crosses to the left chest at the fourth thoracic vertebra. |
The thoracic duct may be visualized by standard lymphangiograms or by nuclear scintigraphy. Often these studies will demonstrate the anatomy of the duct and the level of the lymphatic leakage. They are rarely helpful, however, in the management of these patients and should not be considered a routine part of their evaluation.
 CLINICAL PRESENTATION
CLINICAL PRESENTATIONChylothorax primarily causes symptoms of respiratory insufficiency as the volume of the effusion gradually increases. Most cases of chylothorax are slow in their progression, and symptoms do not occur for several days to several weeks after the initial injury to the duct. Rapid accumulation of chyle with the production of severe respiratory symptoms is uncommon but is occasionally encountered after complete transection of the duct by traumatic or surgical injury. Chyle itself is bacteriostatic, probably because of its high fatty acid content, and symptoms of pleural infection are uncommon. Patients with longstanding chylothorax with loss of significant volumes of chyle often become hypoproteinemic. These patients also often develop lymphopenia from the loss of T lymphocytes in the chyle and may
become relatively immunocompromised. In fact, malnutrition and infection account for the majority of deaths after the development of a chylothorax.
become relatively immunocompromised. In fact, malnutrition and infection account for the majority of deaths after the development of a chylothorax.
Table 27.2 Composition of Chyle | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
MANAGEMENT
The control of chylothorax is thought to occur with the formation of pleural adhesions in the region of the chylous leak, thus preventing the flow of lymph from the thoracic duct or its branches. The management of patients with confirmed chylothorax, therefore, begins with attempts to completely drain the lymphatic fluid from the chest. In some cases, this may be accomplished by thoracentesis, whereas in most patients, with more rapid fluid accumulation, a tube thoracostomy is required. Measures are then instituted to reduce total thoracic duct lymphatic flow, initially using an oral diet containing fats primarily in the form of medium-chain triglycerides, which are absorbed directly into the portal venous system. Patients in whom significant lymphatic flow persists may require total elimination of enteral nutrition, with the institution of intravenous alimentation.
Several clinical reports have suggested that the use of somatostatin or its longer acting synthetic analog octreotide may stop persistent chylous accumulation. The effect of somatostatin on thoracic duct flow is probably secondary to its reduction of splanchnic blood flow and its reduction of intestinal fat absorption, reducing chylomicron synthesis. Somatostatin has generally been administered as an intravenous infusion, using 250 µg/h in adults and 3.5 to 10 µg/kg/h in children. The dose may be increased in stepwise manner to maximal response. Octreotide has generally been administered subcutaneously at doses of 100 µg twice or thrice daily for adults and 10 to 40 µg/kg per day in children (Table 27.3). Children and diabetic adults should be monitored for hyperglycemia or hypoglycemia, and adults are occasionally noted to have cardiac arrhythmias. The safety profile of these drugs, however, appears excellent, and they should be used early in patients with persistent chylous effusions to attempt to avoid protein and fat loss.
The length of time that it is reasonable to persist with conservative therapy is somewhat controversial and depends on the cause of the chylothorax and the volume of lymphatic loss. Some authors suggest intervention in cases of traumatic chylothorax when the daily loss of chyle exceeds 1,500 ml in adults or 100 ml per year of age
in children for a 5-day period, when the output of chyle has not diminished over a 14-day period, or when nutritional complications appear imminent.
in children for a 5-day period, when the output of chyle has not diminished over a 14-day period, or when nutritional complications appear imminent.
Table 27.3 Somatostatin/Octreotide Dose | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||
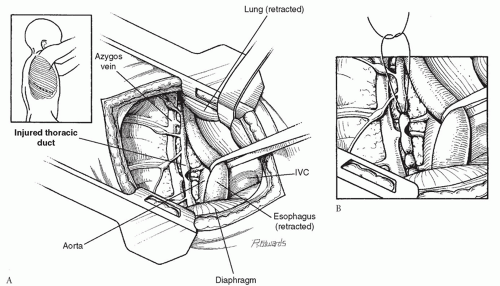
The mortality for patients with chylothorax was approximately 50% until Lampson described mediastinal ligation of the thoracic duct in 1948. This procedure remains the most commonly used operation for persistent chylothorax, although several alternative procedures have been developed in recent years. In patients with unilateral chylous effusions, the chest should be opened on the ipsilateral side, whereas in cases of bilateral effusion, the right chest should be chosen initially. Many authors recommend instillation of 100 to 200 ml of olive oil or cream into the stomach several hours before the operation to increase the fat content of the thoracic lymph and make the area of leakage from the duct itself more easily identifiable. After the induction of general anesthesia with orotracheal intubation, the patient is placed in a full lateral decubitus position. A posterolateral thoracotomy is performed, and the chest is entered through the seventh or eighth intercostal space. If the chylothorax has developed after a previous thoracotomy, the original incision is opened. The mediastinal tissues are examined carefully for the evidence of chylous leak. If such an area is identified, this region should be obliterated with nonabsorbable sutures, occasionally using polytetrafluoroethylene (Teflon) pledgets to compress larger areas of tissue. Whether or not a specific area of leakage is identified and controlled, the main thoracic duct should be ligated as it enters the chest through the aortic hiatus. To accomplish this, the esophagus is encircled and retracted anteriorly, and the tissues between the azygos vein and the descending aorta just cephalad to the aortic hiatus are ligated with nonabsorbable sutures (Fig. 27.3). In most cases, the actual thoracic duct may be identified in this area and ligation performed, whereas in other cases mass ligature of the tissues in this area is accomplished, without direct identification of the duct itself. The chest is closed in layers with a single large chest tube connected to a water seal. A retropleural approach to the thoracic duct has been described. The patient is placed under general anesthesia and positioned prone, and a segment of the right posterior eighth rib is resected after the periosteum is stripped. The posterior mediastinal pleura is dissected bluntly from the chest wall in this region, and the thoracic duct is identified medial to the azygos vein. The duct is obliterated with nonabsorbable suture material, and the incision is closed in layers without drainage.
Patients in whom the source of the chylothorax is suspected to be relatively localized, such as patients developing a chylous effusion after blunt or penetrating thoracic trauma, may benefit by thoracoscopic control of the lymphatic leak. Although the procedure may be performed under local anesthesia in these patients, the use of general anesthesia with unilateral ventilation greatly enhances the exposure of the mediastinum. The patient is placed in a full lateral decubitus position or rolled
slightly forward to facilitate exposure of the posterior mediastinum. The initial trocar for the thoracoscope is placed in the sixth intercostal space in the mid-axillary line. A second trocar is placed posteriorly in the same or adjacent interspace (Fig. 27.4). The residual pleural lymph is aspirated, and the pleura overlying the posterior mediastinum is carefully examined from the level of the inferior pulmonary ligament to the innominate vein. In some cases, a defect in the parietal pleura will be discovered with lymphatic leakage through this region. We have preferred to use metallic clips on this tissue to obliterate the leak, although others have used direct suture ligation (Fig. 27.5). After the pleural leak is controlled in this region, the entire area is flooded with fibrin glue to form a seal over the parietal pleura. In most cases, the inferior pulmonary ligament is then divided with the electrocautery, and the thoracic duct is clipped or ligated at the level of the aortic hiatus. A single chest tube is placed through one of the trocar tracks while the other trocar sites are closed with subcuticular sutures.
slightly forward to facilitate exposure of the posterior mediastinum. The initial trocar for the thoracoscope is placed in the sixth intercostal space in the mid-axillary line. A second trocar is placed posteriorly in the same or adjacent interspace (Fig. 27.4). The residual pleural lymph is aspirated, and the pleura overlying the posterior mediastinum is carefully examined from the level of the inferior pulmonary ligament to the innominate vein. In some cases, a defect in the parietal pleura will be discovered with lymphatic leakage through this region. We have preferred to use metallic clips on this tissue to obliterate the leak, although others have used direct suture ligation (Fig. 27.5). After the pleural leak is controlled in this region, the entire area is flooded with fibrin glue to form a seal over the parietal pleura. In most cases, the inferior pulmonary ligament is then divided with the electrocautery, and the thoracic duct is clipped or ligated at the level of the aortic hiatus. A single chest tube is placed through one of the trocar tracks while the other trocar sites are closed with subcuticular sutures.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree