THE TACHYARRHYTHMIAS
The term tachyarrhythmias typically refers to nonsustained and sustained forms of tachycardia originating from myocardial foci or reentrant circuits. The standard definition of tachycardia is a rhythm that produces a ventricular rate >100 beats per minute. This definition has some limitations in that atrial rates can exceed 100 beats per minute despite a slow ventricular rate. Furthermore, ventricular rates may exceed the baseline sinus rate and be <100 beats per minute but still represent an important “tachycardia” response, as is observed with accelerated ventricular rhythms. Premature complexes (depolarizations) are considered under the category of tachyarrhythmias because they may cause arrhythmia-related symptoms and/or serve as triggering events for more sustained forms of tachycardia.
SYMPTOMS DUE TO TACHYARRHYTHMIAS
Tachyarrhythmias classically produce symptoms of palpitations or racing of the pulse. With premature beats, skipping of the pulse or a pause may be experienced, and patients may even sense slowing of the heart rate or dizziness. A more dramatic irregularity of the pulse is experienced with chaotic rapid rhythms or tachyarrhythmias that originate in the atrium and conduct variably to the ventricles. With rapid tachyarrhythmias, hemodynamic compromise can occur, as can dizziness or syncope due to a decrease in cardiac output or breathlessness due to a marked increase in cardiac filling pressures. Occasionally, chest discomfort may be experienced that mimics symptoms of myocardial ischemia. The underlying cardiac condition typically dictates the severity of symptoms at any specific heart rate. Even patients with normal systolic left ventricular (LV) function may experience severe symptoms if diastolic compliance due to hypertrophy or valvular obstruction is present and a tachycardia develops. Hemodynamic collapse with the development of ventricular fibrillation (VF) can lead to sudden cardiac death (SCD) (Chap. 29).
DIAGNOSTIC TESTS IN EVALUATING TACHYARRHYTHMIAS
In patients who present with nonlife-threatening symptoms such as palpitations or dizziness, electrocardiographic (ECG) confirmation of an arrhythmia with the development of recurrent symptoms is essential. A 24-h Holter monitor should be considered only for patients with daily symptoms. For intermittent symptoms that are of prolonged duration, a patient-activated event monitor can be used to obtain the ECG information without the need for continuous ECG lead attachment and recordings. A patient-activated monitor with a continuously recorded memory loop (“loop recorder”) can be used to document short-lived episodes and the onset of the arrhythmia. This is the preferred monitoring technique for symptomatic patients with less frequent arrhythmia events, but it requires continuous ECG recording. A monitor that automatically triggers to record a fast rhythm can be used to detect asymptomatic arrhythmias. Patients with infrequent, severe symptoms that cannot be identified by intermittent ECG monitoring may receive an implanted loop ECG monitor that provides more extended periods of monitoring and automatic arrhythmia detection (Fig. 16-1).
FIGURE 16-1
Spontaneous termination of atrial fibrillation at the time of a syncopal episode identified from implantable loop ECG recording.
In patients who present with more severe symptoms, such as syncope, outpatient monitoring may be insufficient. In patients with structural heart disease and syncope in whom there is suspicion of ventricular tachycardia (VT), hospitalization and diagnostic electrophysiologic testing are warranted, with strong consideration of an implantable cardioverter/defibrillator (ICD) device. The 12-lead ECG recorded in sinus rhythm should be assessed carefully in patients without structural heart disease for evidence of ST-segment elevation in leads V1 and V2 consistent with Brugada syndrome, QT interval changes consistent with long or short QT syndromes, or a short PR interval and delta wave consistent with Wolff-Parkinson-White (WPW) syndrome. These ECG patterns identify a possible arrhythmogenic substrate that may cause intermittent life-threatening symptoms and warrant further evaluation and therapy. The individual syndromes are discussed in detail later in this chapter.
Monitoring for asymptomatic tachyarrhythmias is indicated in several specific situations. In patients with a suspected tachycardia-induced cardiomyopathy marked by chamber dilation and depression in systolic function, the demonstration of arrhythmia control is essential. Monitoring for asymptomatic ventricular premature complexes (VPCs) and nonsustained VT can be helpful in stratifying the risk of SCD in patients with depressed LV function after myocardial infarction (MI). Finally, in patients with asymptomatic atrial fibrillation (AF), anticoagulation treatment strategies depend on an accurate assessment of the presence of this arrhythmia. The duration of monitoring for asymptomatic arrhythmias may have to be extended to optimize detection capabilities.
A 12-lead ECG recording during the tachycardia can be an important diagnostic tool in identifying the mechanism and origin of a tachycardia to a degree not afforded by one- or two-lead ECG recordings. A 12-lead ECG of the tachyarrhythmia should be recorded and incorporated as a permanent part of the medical record whenever possible. For patients whose arrhythmias are provoked by exercise, an exercise test may provide an opportunity to obtain 12-lead ECG recordings of the arrhythmia and may obviate the need for more extended periods of monitoring.
Many paroxysmal supraventricular tachyarrhythmias are not associated with a significant risk of structural heart disease, and an evaluation for the presence of ischemic heart disease and cardiac function is required infrequently unless dictated by the severity or characteristics of the symptoms. However, in patients with focal or macroreentrant atrial tachycardias (ATs), atrial flutter (AFL), or AF, an evaluation of cardiac chamber size and function and of valve function is warranted. In patients with VT, an echocardiographic assessment of LV and right ventricular (RV) size and function should be the norm. Ventricular tachycardia that occurs in the setting of depressed LV function should raise the suspicion of advanced coronary artery disease (CAD). Ventricular tachycardia in the setting of isolated RV dilation should raise concern about the diagnosis of arrhythmogenic RV cardiomyopathy. Polymorphic VT in the absence of QT prolongation should always raise concern for a potentially unstable ischemic process that may need to be corrected to effect VT control.
MECHANISMS OF TACHYARRHYTHMIAS
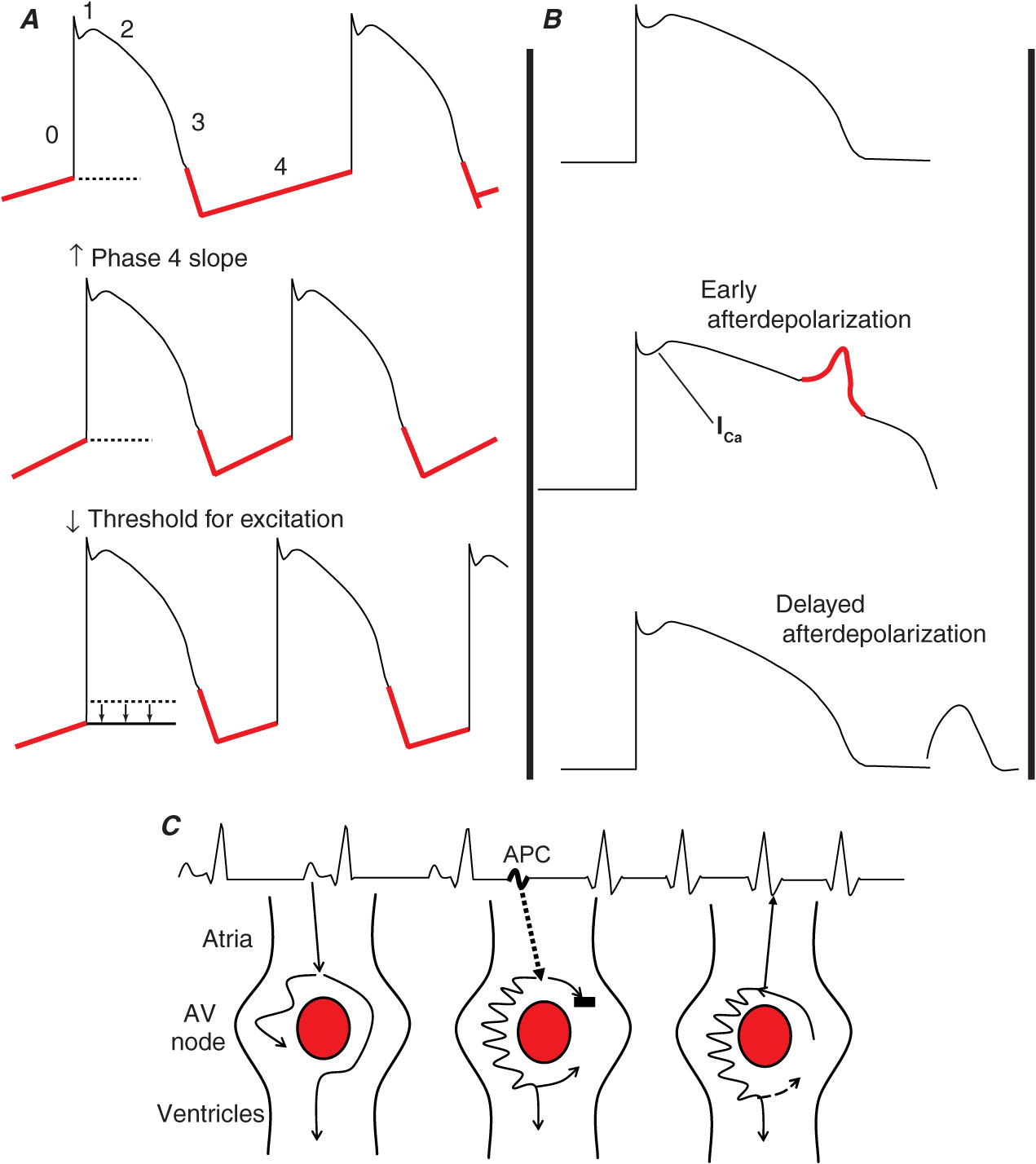
Tachycardias are due to abnormalities of impulse formation and/or abnormalities of impulse propagation (Fig. 16-2).
FIGURE 16-2
Schematic representation of the different mechanisms for arrhythmias. A. Abnormal automaticity due to an increased slope of phase 4 of the action potential or a decrease in the threshold for phase 0. B. Triggered activity due to early afterdepolarizations (EADs) during phase 3 of the action potential due to alteration of plateau currents or delayed afterdepolarizations (DADs) during phase 4 of the action potential due to intracellular calcium accumulation. C. Reentry with basic requirements of two pathways that have heterogeneous electrophysiologic properties which allows conduction to block in one pathway and propagate slowly in the other, allowing for sufficient delay so that the blocked site has time for recovery to allow for reentry or circus movement tachycardia. Shown is a typical schema for reentry in the AV node. AV, atrioventricular; APC, atrial premature complex.
Abnormalities in impulse formation
An increase in automaticity normally causes an increase in sinus rate and sinus tachycardia (Fig. 16-2A). Abnormal automaticity is due to an increase in the slope of phase 4 depolarization or a reduced threshold for action potential depolarization in myocardium other than the sinus node. Abnormal automaticity is thought to be responsible for most atrial premature complexes (APCs) and VPCs and some ATs. Pacing does not provoke automatic rhythms. Less commonly, abnormal impulse formation is due to triggered activity. Triggered activity is related to cellular afterdepolarizations that occur at the end of the action potential, during phase 3, and are referred to as early afterdepolarizations; when they occur after the action potential, during phase 4, they are referred to as late afterdepolarizations. Afterdepolarizations are attributable to an increase in intracellular calcium accumulation. If sufficient afterdepolarization amplitude is achieved, repeated myocardial depolarization and a tachycardic response can occur. Early afterdepolarizations may be responsible for the VPCs that trigger the polymorphic ventricular arrhythmia known as torsades des pointes (TDP). Late afterdepolarizations are thought to be responsible for atrial, junctional, and fascicular tachyarrhythmias caused by digoxin toxicity and also appear to be the basis for catecholamine-sensitive VT originating in the outflow tract. In contrast to automatic tachycardias, those due to triggered activity (Fig. 16-2B) frequently can be provoked with pacing maneuvers.
Abnormalities in impulse propagation
Reentry is due to inhomogeneities in myocardial conduction and/or recovery properties. The presence of a unidirectional block with slow conduction to allow for retrograde recovery of the blocked myocardium allows the formation of a circuit that, if perpetuated, can sustain a tachycardia (Fig. 16-2C). These inhomogeneities are somewhat inherent but are minimized in normal myocardial activation/recovery. The inhomogeneities can be exaggerated by the presence of extra pathways, as occurs with the WPW syndrome; generalized genetically determined myocardial ion channel abnormalities, as occur with long QT syndrome (LQTS); or the interruption of normal myocardial patterns of activation due to the development of fibrosis.
Reentry appears to be the basis for most abnormal sustained supraventricular tachycardias (SVTs) and VTs. In general, reentry can be anatomically driven (fixed) based on the presence of “extra” pathways, natural anatomic barriers of conduction such as the crista terminalis, the vertical crest on the interior wall of the right atrium that separates the nontrabeculated posterior right atrium from the rest of the trabeculated right atrium located lateral to the structure, and/or extensive fibrosis created by underlying myocardial disease. This form of reentry seems to be more stable and results in a tachycardia that has a uniform (often monomorphic), repetitive appearance. Other forms of reentry appear to be more functional and are more dependent on dynamic changes in electrophysiologic properties of the myocardium. These tachycardias tend to be more unstable and may result in tachycardias that have a polymorphic appearance. Two classic examples of reentry that are primarily functional are VF due to acute myocardial ischemia and polymorphic VT in patients with a genetically determined ion channel abnormality such as Brugada syndrome, LQTS, or catecholaminergic polymorphic VT.
SUPRAVENTRICULAR TACHYARRHYTHMIAS
ATRIAL PREMATURE COMPLEXES
Atrial premature complexes are the most common arrhythmia identified during extended ECG monitoring. The incidence of APCs frequently increases with age and with the presence of structural heart diseases. Atrial premature complexes typically are asymptomatic, although some patients experience palpitations or an irregularity of the pulse.
ECG diagnosis of APCs
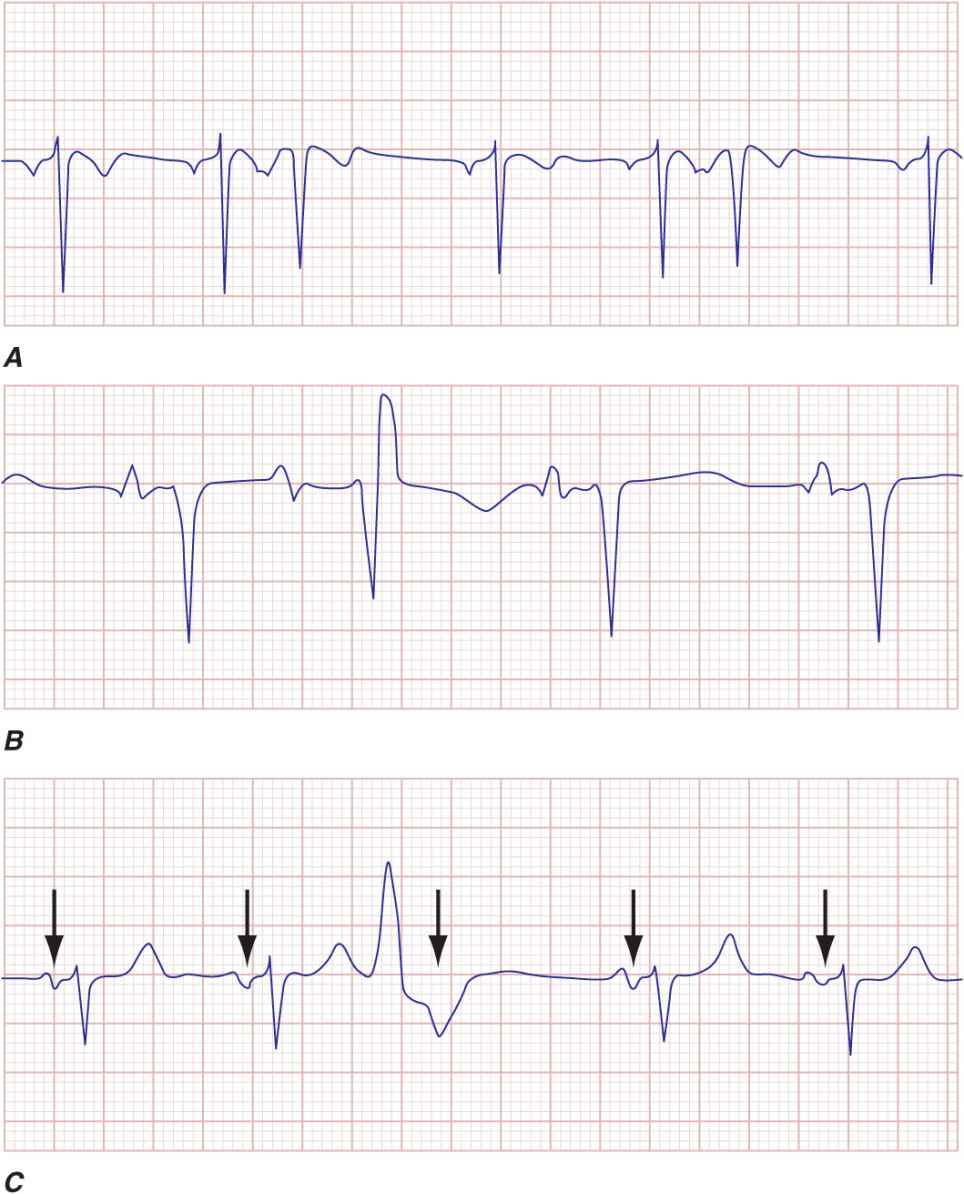
The ECG diagnosis of APCs is based on the identification of a P wave that occurs before the anticipated sinus beat (Fig. 16-3A and B). The source of the APC appears to parallel the typical sites of origin for ATs. The P-wave contour typically differs from that noted during sinus rhythm, although APCs from the right atrial appendage, superior vena cava (SVC), and superior aspect of the crista terminalis in the region of the sinus node may mimic the sinus P-wave morphology. In response to an APC, the PR interval lengthens, although APCs that originate near the atrioventricular (AV) nodal region may actually have a shorter PR interval because the atrial conduction time to the junction is shortened. A very early APC may not conduct to the ventricle and can create a pulse irregularity that may be perceived as a pause or “dropped beat.” If the APC conducts rapidly through the AV node, a partially recovered His-Purkinje system will be encountered and a QRS pattern consistent with a right or left bundle branch block may occur. This wide QRS pattern and the failure to recognize the preceding P wave may result in misdiagnosis of VPCs. APCs characteristically reset the sinus node. The resulting sum of the pre- and post-APC RR interval is less than two sinus PP intervals.
FIGURE 16-3
Atrial and ventricular premature complexes (APCs, VPCs). The APC resets the sinus node, and no compensatory pause is present A. even when conducted aberrantly in the ventricles with a bundle branch block-type QRS pattern B. VPCs tend not to reset the sinus activity (arrows) and will demonstrate a full compensatory pause C.
TREATMENT Atrial Premature Complexes
Atrial premature complexes generally do not require intervention. For extremely symptomatic patients who do not respond to explanation and reassurance, an attempt can be made to suppress the APCs with pharmacologic agents. The repetitive focus can even be targeted for catheter ablation. Beta blockers may be tried. Of note, these agents may uncommonly exacerbate symptoms if AV block occurs with the APC and irregularity of the pulse consequently becomes more profound. The use of class IC antiarrhythmic agents may eliminate the APCs but should be avoided if structural heart disease is present.
JUNCTIONAL PREMATURE COMPLEXES
Junctional premature complexes are extremely uncommon. The complexes originate from the AV node and His bundle region and may produce retrograde atrial activation with the P wave distorting the initial or terminal portions of the QRS complex, producing pseudo Q or S waves in leads II, III, and aVF. Extrasystoles originating in the bundle of His that do not conduct to the ventricle and also block the atria can produce unexplained surface ECG PR prolongation that does not follow a typical Wenckebach periodicity (i.e., gradual PR prolongation culminating in atrial activity that fails to conduct to the ventricles). Intracardiac recordings frequently can identify a His depolarization, thus identifying the origin of the complex to the AV junction. Symptomatic patients typically may be treated with beta blockers or, if there is no structural heart disease, class IC antiarrhythmic agents.
SINUS TACHYCARDIA
Physiologic sinus tachycardia represents a normal or appropriate response to physiologic stress, such as that which occurs with exercise, anxiety, or fever. Pathologic conditions such as thyrotoxicosis, anemia, and hypotension also may produce sinus tachycardia. It is important to distinguish sinus tachycardia from other SVTs. Sinus tachycardia will produce a P-wave contour consistent with its origin from the sinus node located in the superior-lateral and posterior aspect of the right atrium. The P wave is upright in leads II, III, and aVF and negative in lead aVR. The P-wave morphology in lead V1 characteristically has a biphasic, positive/negative contour. Onset of sinus tachycardia is gradual, and in response to carotid sinus pressure there may be some modest and transient slowing but no abrupt termination. Importantly, the diagnosis should not be based on the PR interval or the presence of a P wave before every QRS complex. The PR interval and the presence of 1:1 AV conduction properties are determined by AV nodal and His-Purkinje conduction; therefore, the PR interval can be dramatically prolonged while sinus tachycardia remains the atrial mechanism.
TREATMENT Physiologic Sinus Tachycardia
Treatment of physiologic sinus tachycardia is directed at the underlying condition causing the tachycardia response. Uncommonly, beta blockers are used to minimize the tachycardia response if it is determined to be potentially harmful, as may occur in a patient with ischemic heart disease and rate-related anginal symptoms.
Inappropriate sinus tachycardia represents an uncommon but important medical condition in which the heart rate increases either spontaneously or out of proportion to the degree of physiologic stress/exercise. Dizziness and even frank syncope often accompany the sinus tachycardia and symptoms of palpitations. The syndrome can be quite disabling. Associated symptoms of chest pain, headaches, and gastrointestinal upset are common. In many patients, the syndrome occurs after a viral illness and may resolve spontaneously over the course of 3–12 months, suggesting a postviral dysautonomia.
Excluding the diagnosis of an automatic AT that originates in the region of the sinus node can be difficult and may require invasive electrophysiologic evaluation. Frequently, patients are misdiagnosed as having an anxiety disorder with physiologic sinus tachycardia.
TREATMENT Inappropriate Sinus Tachycardia
For symptomatic patients, maintaining an increased state of hydration, salt loading, and careful titration of beta blockers to the maximum tolerated dose, administered in divided doses, frequently minimize symptoms. For severely symptomatic patients who are intolerant of or unresponsive to beta blockers, catheter ablation directed at modifying the sinus node may be effective. Because of the high recurrence rate after ablation and the frequent need for atrial pacing therapy, this intervention remains second-line treatment.
ATRIAL FIBRILLATION
(Fig. 16-4) Atrial fibrillation is the most common sustained arrhythmia. It is marked by disorganized, rapid, and irregular atrial activation. The ventricular response to the rapid atrial activation is also irregular. In an untreated patient, the ventricular rate also tends to be rapid and is entirely dependent on the conduction properties of the AV junction. Although typically the rate will vary between 120 and 160 beats per minute, in some patients it can be >200 beats per minute. In other patients, because of heightened vagal tone or intrinsic AV nodal conduction properties, the ventricular response is <100 beats per minute and occasionally even profoundly slow. The mechanism for AF initiation and maintenance, although still debated, appears to be a complex interaction between drivers responsible for the initiation and the complex anatomic atrial substrate that promotes the maintenance of multiple wavelets of (micro)reentry. The drivers appear to originate predominantly from the atrialized musculature that enters the pulmonary veins and represent either focal abnormal automaticity or triggered firing that is somewhat modulated by autonomic influences. Sustained forms of microreentry as drivers also have been documented around the orifice of pulmonary veins; nonpulmonary vein drivers also have been demonstrated. The role these drivers play in maintaining the tachycardias may be significant and may explain the success of pulmonary vein isolation procedures in eliminating more chronic or persistent forms of AF.
FIGURE 16-4
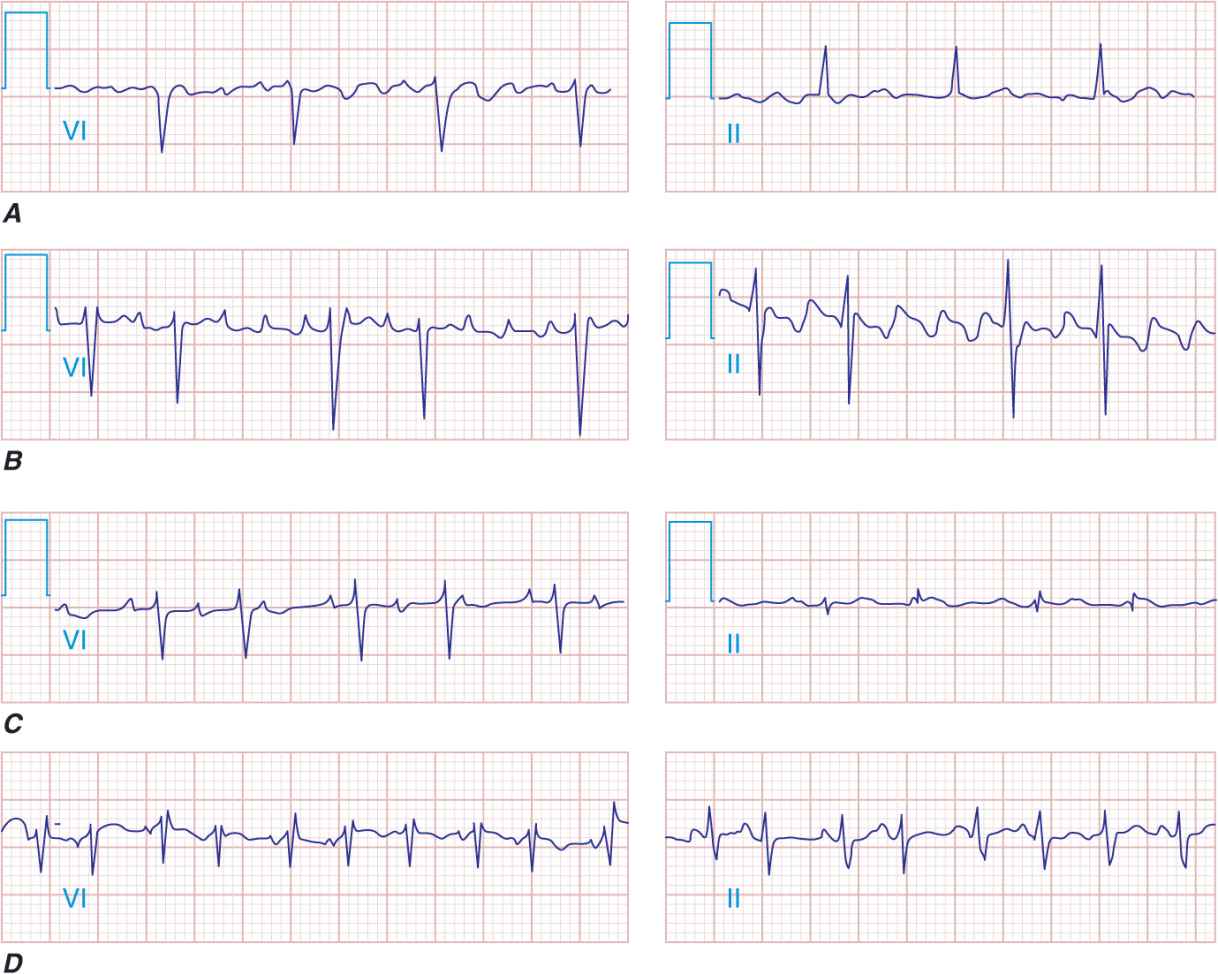
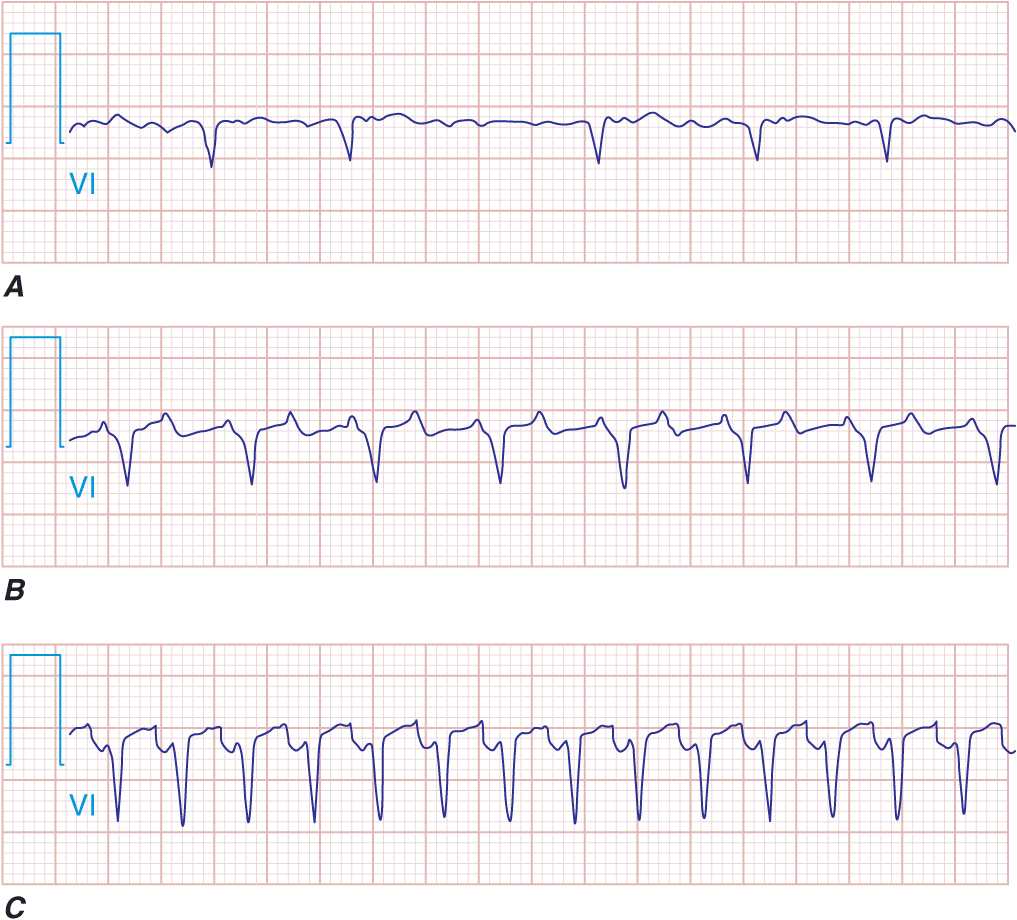
Supraventricular tachycardias with irregular ventricular rates. Atrial fibrillation (A), atrial flutter (B), atrial tachycardia (C), and multifocal atrial tachycardia (MAT; D) are shown. The characteristics of the atrial activity with respect to the morphology and rate provide the clues to the diagnosis. The variable ventricular response to the atrial flutter and the atrial tachycardia suggest a Wenckebach-type periodicity.
Although AF is common in the adult population, it is extremely unusual in children unless structural heart disease is present or there is another arrhythmia that precipitates the AF, such as paroxysmal SVT in patients with WPW syndrome. The incidence of AF increases with age such that >5% of the adult population over 70 will experience the arrhythmia. As many patients are asymptomatic with AF, it is anticipated that the overall incidence, particularly that noted in the elderly, may be more than double the previously reported rates. Occasionally, AF appears to have a well-defined etiology, such as acute hyperthyroidism, an acute vagotonic episode, or acute alcohol intoxication. Acute AF is particularly common during the acute or early recovery phase of major vascular, abdominal, and thoracic surgery, in which case autonomic fluxes and/or direct mechanical irritation potentiate the arrhythmia. AF also may be triggered by other supraventricular tachycardias, such as AV nodal reentrant tachycardia (AVNRT), and elimination of these arrhythmias may prevent AF recurrence.
AF has clinical importance related to (1) the loss of atrial contractility, (2) the inappropriate fast ventricular response, and (3) the loss of atrial appendage contractility and emptying leading to the risk of clot formation and subsequent thromboembolic events.
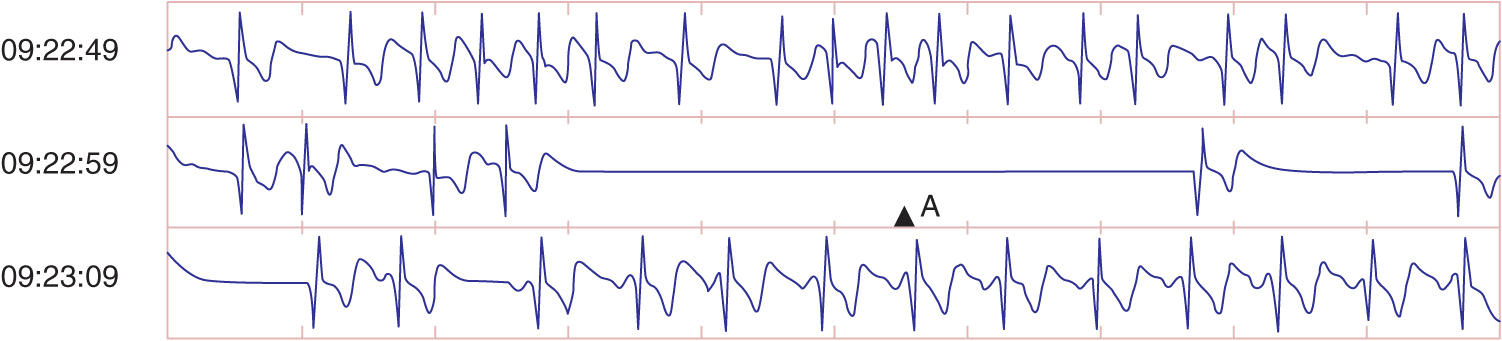
Symptoms from AF vary dramatically. Many patients are asymptomatic and have no apparent hemodynamic consequences from the development of AF. Other patients experience only minor palpitations or sense irregularity of the pulse. Many patients, however, experience severe palpitations. The hemodynamic effect in patients can be quite dramatic, depending on the need for normal atrial contractility and the ventricular response. Hypotension, pulmonary congestion, and anginal symptoms may be severe in some patients. In patients with LV diastolic dysfunction that occurs with hypertension, hypertrophic cardiomyopathy, or obstructive aortic valvular disease, symptoms may be even more dramatic, especially if the ventricular rate does not permit adequate ventricular filling. Exercise intolerance and easy fatigability are the hallmarks of poor rate control with exertion. Occasionally, the only manifestation of AF is severe dizziness or syncope associated with the pause that occurs upon termination of AF before sinus rhythm resumes (Fig. 16-1).
The ECG in AF is characterized by the lack of organized atrial activity and the irregularly irregular ventricular response. Occasionally, one needs to record from multiple ECG leads simultaneously to identify the chaotic continuous atrial activation. Lead V1 frequently shows the appearance of organized atrial activity that mimics AFL. This occurs because the crista terminalis serves as an effective anatomic barrier to electrical conduction, and the activation of the lateral right atrium may be represented by a more uniform activation wave front that originates over the roof of the right atrium. ECG assessment of the PP interval (<200 ms) and the chaotic P-wave morphology in the remaining ECG leads will confirm the presence of AF.
Evaluation of a patient with AF should include a search for a reversible cause of the arrhythmia, such as hyperthyroidism or anemia. An echocardiogram should be performed to determine whether there is structural heart disease. Persistent or labile hypertension should be identified and treated.
Treatment for AF must take into account the clinical situation in which the arrhythmia is encountered, the chronicity of the AF, the status of the patient’s level of anticoagulation, risk factors for stroke, the patient’s symptoms, the hemodynamic impact of the AF, and the ventricular rate.
ACUTE RATE CONTROL In the absence of hemodynamic compromise that might warrant emergent cardioversion to terminate the AF, the initial goals of therapy are to (1) establish control of the ventricular rate and (2) address anticoagulation status and begin IV heparin treatment if the duration of AF is >12 h and risk factors for stroke with AF are present (Table 16-1). Ventricular rate control for acute AF is best established with beta blockers and/or the calcium channel blocking agents verapamil and diltiazem. The route of administration and dose will be dictated by the ventricular rate and clinical status. Digoxin may add to the rate-controlling benefit of the other agents but is uncommonly used as a stand-alone agent, especially in acute AF.
TABLE 16-1
RISK FACTORS FOR STROKE IN ATRIAL FIBRILLATION
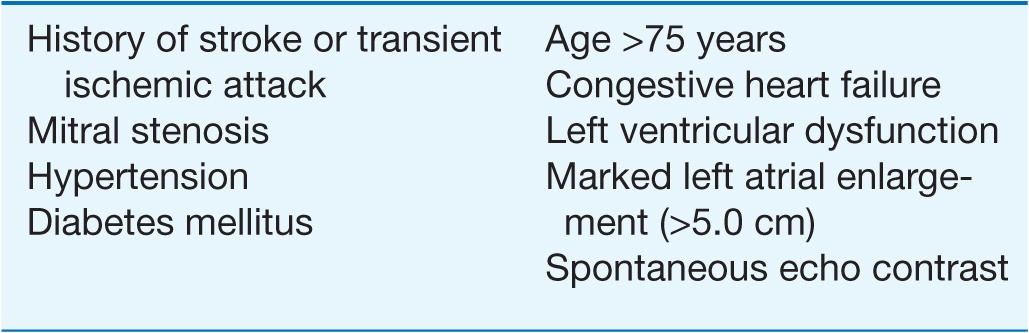
Anticoagulation is of particular importance in patients who have known risk factors for stroke associated with AF. Factors associated with the highest risk of stroke include a history of stroke, transient ischemic attack (TIA) or systemic embolism, and the presence of rheumatic mitral stenosis. Other identified risk factors include age >65 years, history of congestive heart failure (CHF), diabetes mellitus, hypertension, LV dysfunction, and evidence of marked left atrial enlargement (>5.0 cm). Chronic anticoagulation with warfarin targeted to achieve an international normalized ratio (INR) between 2.0 and 3.0 is recommended in patients with persistent or frequent and long-lived paroxysmal AF and risk factors. If patients have not been adequately anticoagulated and the AF is more than 24–48 h in duration, a transesophageal echocardiogram (TEE) can be performed to exclude the presence of a left atrial thrombus that might dislodge with the attempted restoration of sinus rhythm with either nonpharmacologic or pharmacologic therapy. Anticoagulation must be instituted coincident with the TEE and maintained for at least 1 month after restoration of sinus rhythm if the duration of AF has been prolonged or is unknown. Heparin is maintained routinely until the INR is 1.8 with the administration of warfarin after the TEE. For patients who do not warrant early cardioversion of AF, anticoagulation should be maintained for at least 3 weeks with the INR confirmed to be >1.8 on at least two separate occasions before attempts at cardioversion.
Termination of AF acutely may be warranted on the basis of clinical parameters and/or hemodynamic status. Confirmation of appropriate anticoagulation status as described earlier in the chapter must be documented unless symptoms and clinical status warrant emergent intervention. Direct current transthoracic cardioversion during short-acting anesthesia is a reliable way to terminate AF. Conversion rates using a 200-J biphasic shock delivered synchronously with the QRS complex typically are >90%. Pharmacologic therapy to terminate AF is less reliable. Oral and/or IV administration of amiodarone or procainamide has only modest success. The acute IV administration of ibutilide appears to be somewhat more effective and may be used in selected patients to facilitate termination with direct current (DC) cardioversion (Tables 16-2 and 16-3).
TABLE 16-2
COMMONLY USED ANTIARRHYTHMIC AGENTS—INTRAVENOUS DOSE RANGE/PRIMARY INDICATION
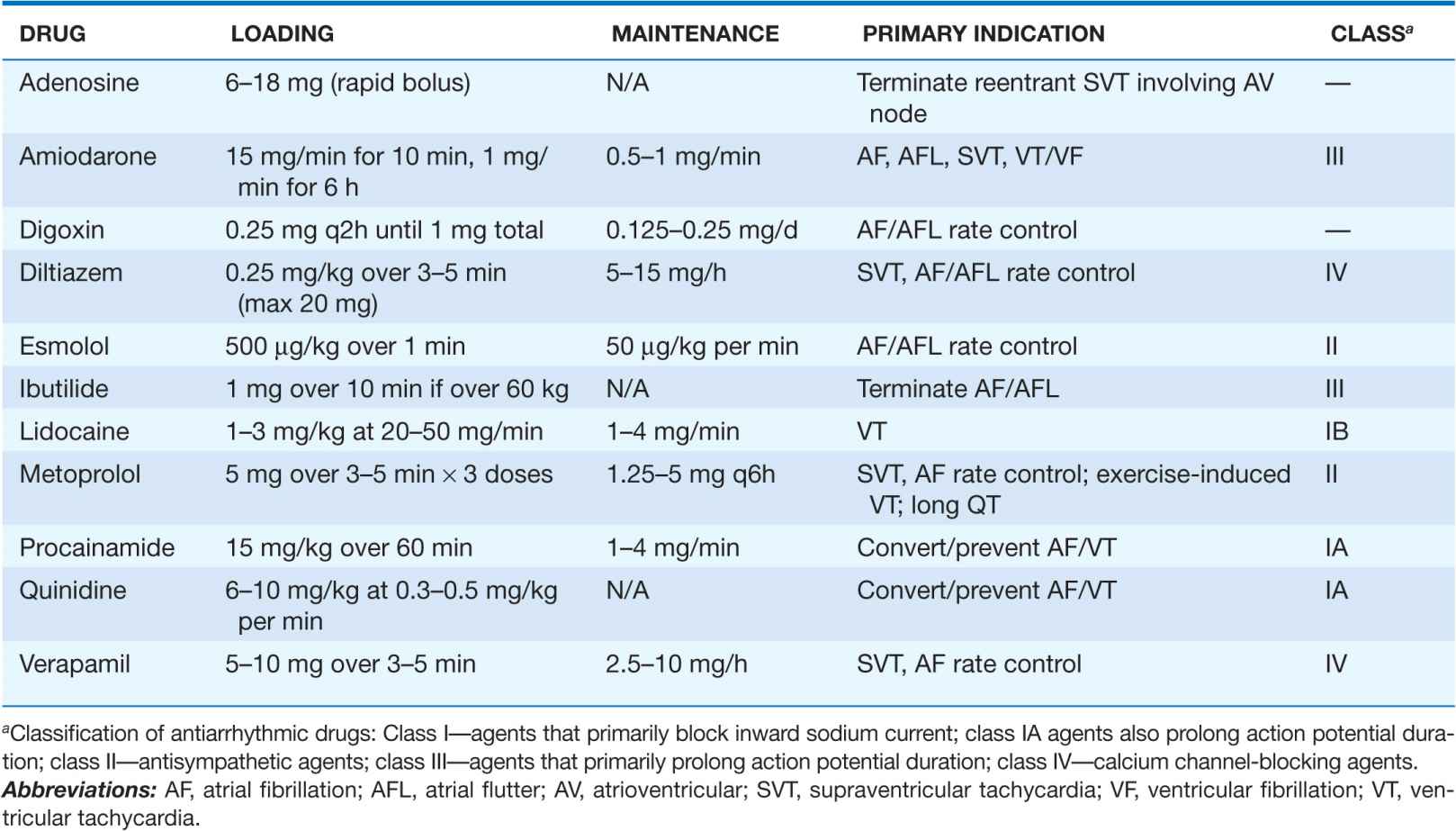
TABLE 16-3
COMMONLY USED ANTIARRHYTHMIC AGENTS: CHRONIC ORAL DOSING/PRIMARY INDICATIONS
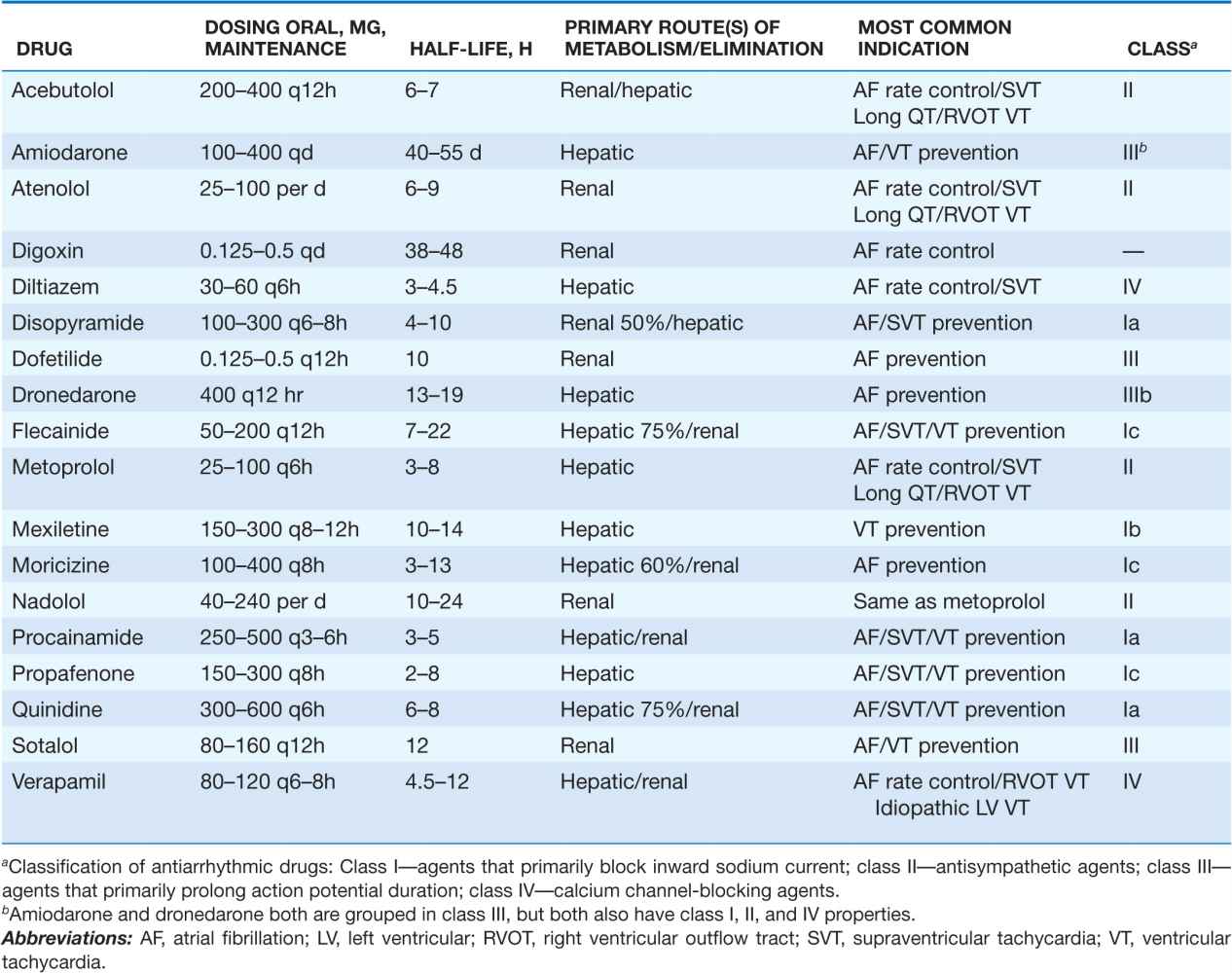
Pharmacologic therapy to maintain sinus rhythm can be instituted once sinus rhythm has been established or in anticipation of cardioversion to attempt to maintain sinus rhythm (Table 16-3). A single episode of AF may not warrant any intervention or only a short course of beta blocker therapy. To prevent recurrent AF unresponsive to beta blockade, a trial of antiarrhythmic therapy may be warranted, particularly if the AF is associated with rapid rates and/or significant symptoms. The selection of antiarrhythmic agents should be dictated primarily by the presence or absence of CAD, depressed LV function not attributable to a reversible tachycardia-induced cardiomyopathy, and/or severe hypertension with evidence of marked LV hypertrophy. The presence of any significant structural heart disease typically narrows treatment to the use of sotalol, amiodarone, dofetilide, or dronedarone. Severely depressed LV function with heart failure symptoms precludes the use of dronedarone and may limit sotalol therapy. Owing to the risk of QT prolongation and polymorphic VT, sotalol and dofetilide have to be initiated in the hospital in most cases.
In patients without evidence of structural heart disease or hypertensive heart disease without evidence of severe hypertrophy, the use of the class IC antiarrhythmic agents flecainide or propafenone appears to be well tolerated and does not have significant proarrhythmia risk. It is important to recognize that no drug is uniformly effective, and arrhythmia recurrence should be anticipated in over one-half of the patients during long-term follow-up regardless of the type and number of agents tried. It is also important to recognize that although the maintenance of sinus rhythm has been associated with improved long-term survival, the survival outcome of patients randomized to the pharmacologic maintenance of sinus rhythm was not superior to that of patients treated with rate control and anticoagulation in the AFFIRM and RACE trials. The AFFIRM and RACE trials compared outcome with respect to survival and thromboembolic events in patients with AF and risk factors for stroke using the two treatment strategies. It is believed that the poor outcome related to pharmacologic therapy used to maintain sinus rhythm was primarily due to the common inefficacy of such drug therapy and an increased incidence of asymptomatic AF. Many of the drugs used for rhythm control, including sotalol, amiodarone, propafenone, dronedarone, and flecainide, enhance slowing of AV nodal conduction. The absence of symptoms frequently leads to stopping anticoagulant therapy, and asymptomatic AF without anticoagulation increases stroke risk. Any consideration for stopping anticoagulation therefore must be accompanied by a prolonged period of ECG monitoring to document asymptomatic AF. It is also recommended that patients participate in monitoring by learning to take their pulse on a twice-daily basis and reliably identify its regularity if discontinuing anticoagulant therapy is contemplated seriously.
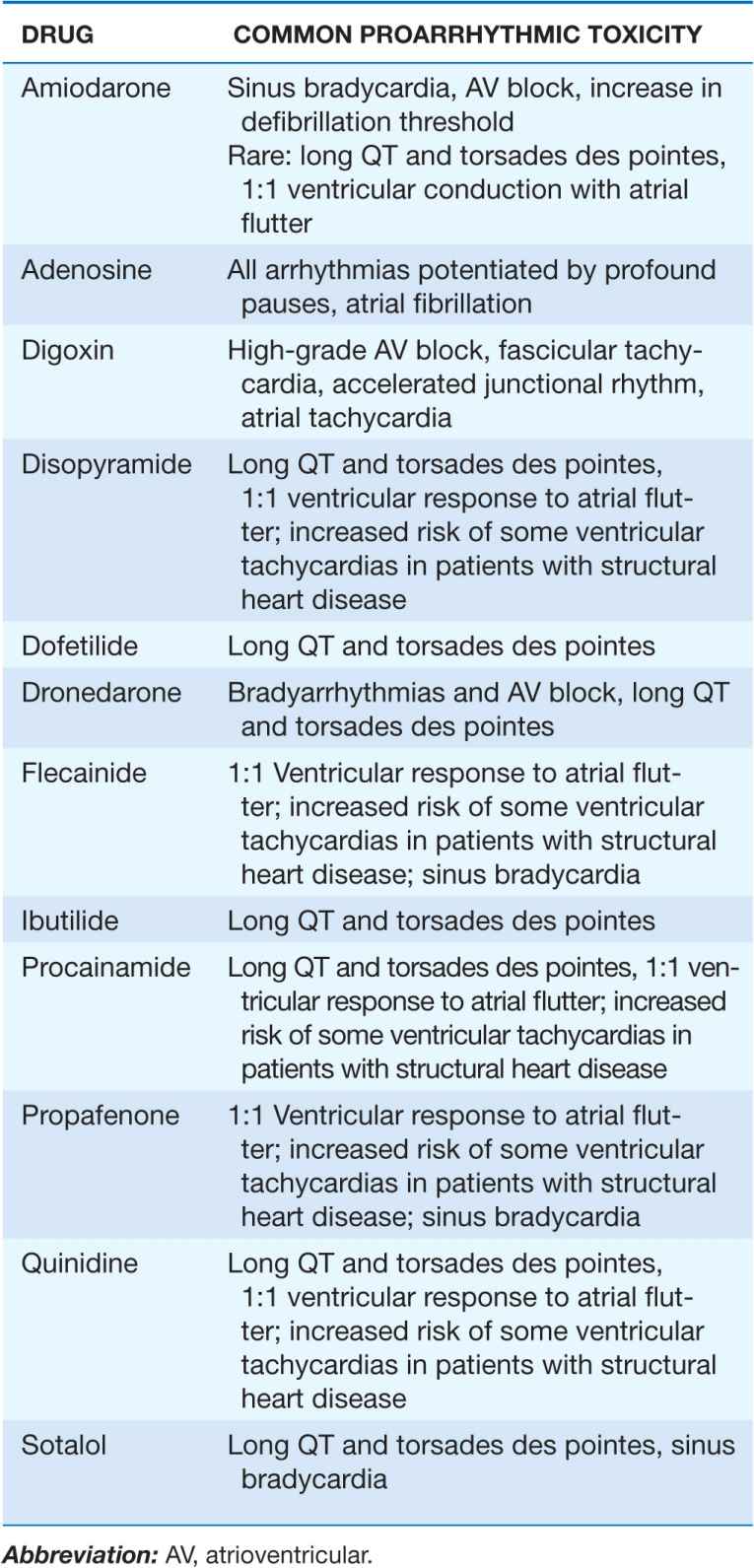
It is clear that to reduce the risk of drug-induced complications in treating AF, a thorough understanding of the drug planned to be used is critical—its dosing, metabolism, and common side effects and important drug-drug interactions. This information has been summarized in Tables 16-2, 16-3, 16-4, and 16-5 and serves as a starting point for a more complete review. In using antiarrhythmic agents that slow atrial conduction, strong consideration should be given to adding a beta blocker or a calcium channel blocker (verapamil or diltiazem) to the treatment regimen. This should help avoid a rapid ventricular response if AF is converted to “slow” AFL with the drug therapy (Fig. 16-5).
FIGURE 16-5
Atrial fibrillation. A. Transitions to “slow” atrial flutter during antiarrhythmic drug therapy. B. A rapid ventricular response with 1:1 atrioventricular conduction occurred with exercise, leading to C. symptoms of dizziness.
TABLE 16-4
COMMON NONARRHYTHMIC TOXICITY OF MOST FREQUENTLY USED ANTIARRHYTHMIC AGENTS
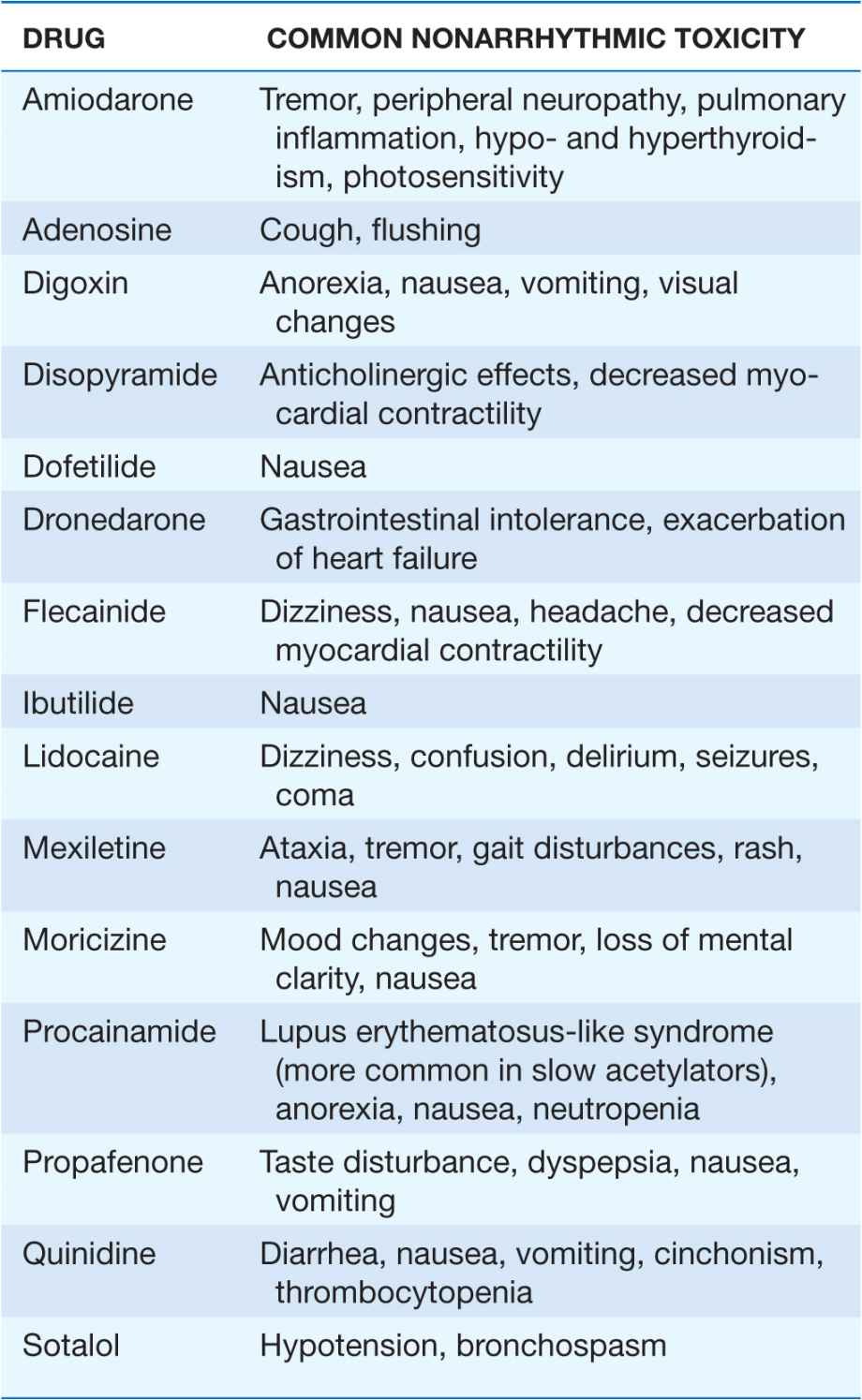
TABLE 16-5
PROARRHYTHMIC MANIFESTATIONS OF MOST FREQUENTLY USED ANTIARRHYTHMIC AGENTS
CHRONIC RATE CONTROL This is an option in patients who are asymptomatic or symptomatic due to the resulting tachycardia. Rate control is frequently difficult to achieve in patients who have paroxysmal AF. In patients with more persistent forms of AF, rate control with beta blockers, the calcium channel blockers diltiazem and verapamil, and/or digoxin frequently can be achieved. Using the drugs in combination may avoid some of the common side effects seen with high-dose monotherapy. An effort should be made to document the adequacy of rate control to reduce the risk of a tachycardia-induced cardiomyopathy. Heart rates >80 beats/min at rest or 100 beats/min with very modest physical activity are indications that rate control may be inadequate in persistent AF. Extended periods of ECG monitoring and assessment of heart rate with exercise should be considered.
In patients with symptoms resulting from inadequate rate control with pharmacologic therapy or worsening LV function due to the persistent tachycardia, ablative therapy to attempt to eliminate atrial fibrillation, or an AV junction ablation can be performed. The AV junction ablation must be coupled with the implantation of an activity sensor pacemaker to maintain a physiologic range of heart rates. Recent evidence that RV pacing can occasionally modestly depress LV function should be taken into consideration in identifying which patients are appropriate candidates for the “ablate and pace” treatment strategy. Occasionally, biventricular pacing may be used to minimize the degree of dyssynchronization that can occur with RV apical pacing alone. Rate control treatment options must be coupled with chronic anticoagulation therapy in all cases. Trials evaluating the elimination of embolic risk by elimination or isolation of the left atrial appendage or by endovascular insertion of a left atrial appendage-occluding device may provide other treatment options that can eliminate the need for chronic anticoagulation.
CATHETER AND SURGICAL ABLATIVE THERAPY TO PREVENT RECURRENT AF Although the optimum ablation strategy has not been defined, most ablation strategies incorporate techniques that isolate the atrial muscle sleeves entering the pulmonary veins; these muscle sleeves have been identified as the source of the majority of triggers responsible for the initiation of AF. Ablation therapy is currently considered an alternative to additional pharmacologic therapy trials in patients with recurrent symptomatic AF or AF associated with poor rate control who have failed an initial attempt at rhythm control with pharmacologic management. Ablative therapy appears superior to additional pharmacologic treatment aimed at rhythm control in this setting. Elimination of AF in 50–80% of patients with a catheter-based ablation procedure should be anticipated, depending on the chronicity of the AF, with additional patients becoming responsive to previously ineffective medications.
Catheter ablative therapy also holds promise in patients with more persistent forms of AF and even those with severe atrial dilation. Its confirmed efficacy suggests an important alternative to His bundle ablation and pacemaker insertion in many patients. Serious risks related to the left atrial ablation procedure, albeit low (overall 2–4%), include pulmonary vein stenosis, atrioesophageal fistula, systemic embolic events, perforation/tamponade, and phrenic nerve injury.
Surgical ablation of AF is typically performed at the time of other cardiac valve or coronary artery surgery and, less commonly, as a stand-alone procedure. The surgical Cox-Maze procedure is designed to interrupt all macroreentrant circuits that might potentially develop in the atria, thereby precluding the ability of the atria to fibrillate. In an attempt to simplify the operation, the multiple incisions of the traditional Cox-Maze procedure have been replaced with linear lines of ablation and pulmonary vein isolation using a variety of energy sources.
Severity of AF symptoms and difficulties in rate and/or rhythm control with pharmacologic therapy frequently dictate the optimum AF treatment strategy. Similar to the approach with pharmacologic rhythm control, a cautious approach to eliminating anticoagulant therapy is recommended after catheter or surgical ablation. Careful ECG monitoring for asymptomatic AF, particularly in patients with multiple risk factors for stroke, should be considered until guidelines are firmly established. If the left atrial appendage has been removed surgically, the threshold for stopping anticoagulation may be lowered. Antiarrhythmic therapy typically can be discontinued after catheter or surgical ablation of AF. However, in selected patients, satisfactory AF control may require maintenance of previously ineffective drug therapy after the ablation intervention.
ATRIAL FLUTTER AND MACROREENTRANT ATRIAL TACHYCARDIAS
Macroreentrant arrhythmias involving the atrial myocardium are referred to collectively as AFL. The terms AFL and macroreentrant AT frequently are used interchangeably, with both denoting a nonfocal source of an atrial arrhythmia. The typical or most common AFL circuit rotates in a clockwise or counterclockwise direction in the right atrium around the tricuspid valve annulus. The posterior boundary of the right AFL circuit is defined by the crista terminalis, the eustachian ridge, and the inferior and superior vena cavae. Counterclockwise right AFL represents ~80% of all AFL with superiorly directed activation of the interatrial septum, which produces the saw-toothed appearance of the P waves in ECG leads II, III, and aVF. Clockwise rotation of the same right atrial circuit produces predominantly positive P waves in leads II, III, and aVF (Fig. 16-4). Macroreentrant left AFL also may develop, albeit much less commonly. This type of arrhythmia may be the sequela of surgical or catheter-based ablation procedures that create large anatomic barriers or promote slowing of conduction in the left atrium, especially around the mitral valve annulus or partially disconnected pulmonary veins. Atypical AFL or macro-reentrant AT can also develop around incisions created during surgery for valvular or congenital heart disease or in and/or around large areas of atrial fibrosis.
Classic or typical right AFL has an atrial rate of 260–300 beats per minute with a ventricular response that tends to be 2:1, or typically 130–150 beats per minute. In the setting of severe atrial conduction disease and or antiarrhythmic drug therapy, the atrial rate can slow to <200 beats per minute. In this setting, a 1:1 rapid ventricular response may occur, particularly with exertion, and produce adverse hemodynamic effects (Fig. 16-5). Atypical AFL or macroreentrant AT related to prior surgical incisions and atrial fibrosis demonstrates less predictability in terms of the atrial rate and is more likely to demonstrate slower rates that overlap with those identified with focal atrial tachycardias.
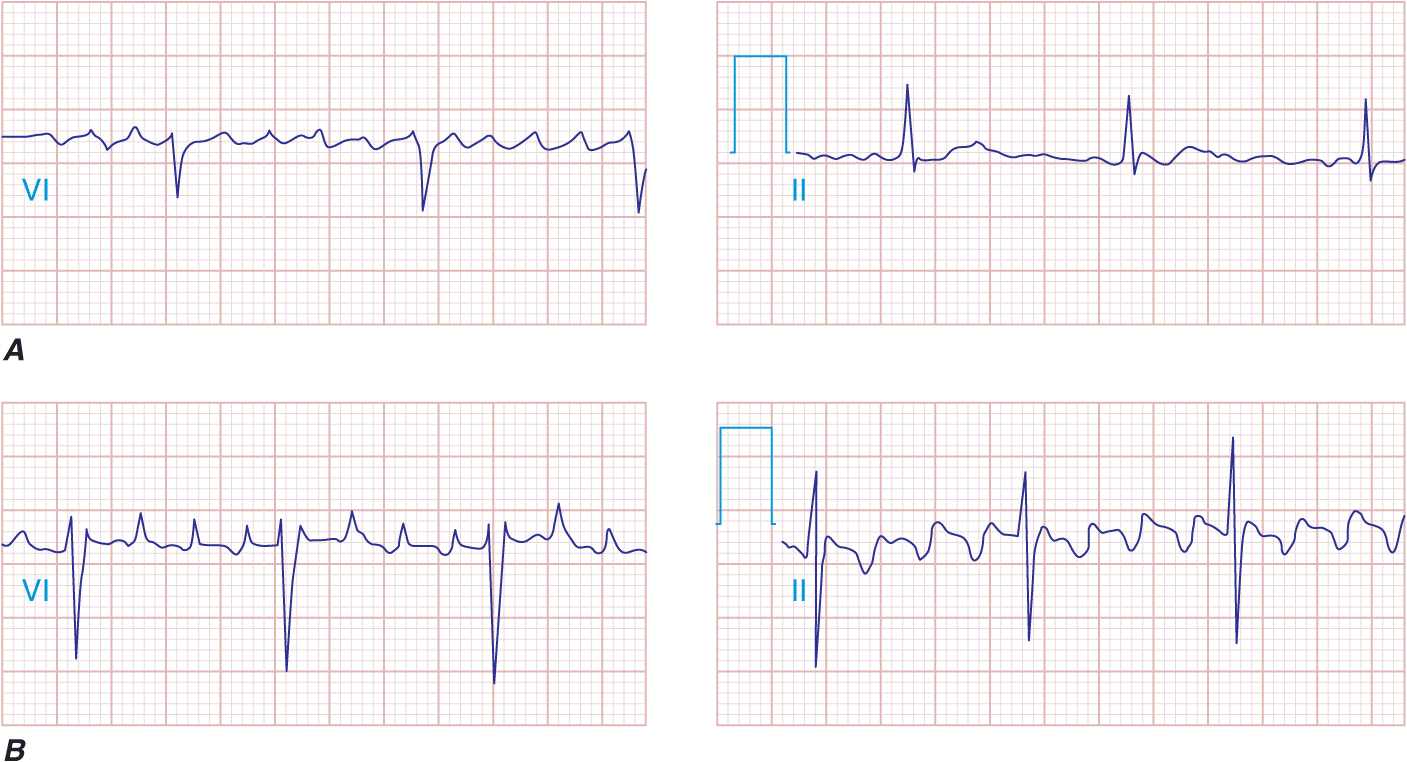
Because lead V1 is frequently monitored in a hospitalized patient, coarse AF may be misdiagnosed as AFL. This occurs because in both typical right AFL and coarse AF the crista terminalis in the right atrium may serve as an effective anatomic barrier. The free wall of the right atrium, whose electrical depolarization is best reflected on the body surface by lead V1, may demonstrate a uniform wave front of atrial activation in both conditions. The timing of atrial activation is much more rapid in AF and always demonstrates variable atrial intervals with some intervals between defined P waves <200 ms (Fig. 16-6). A review of the other ECG leads demonstrates the disorganized atrial depolarization that is characteristic of AF. Frequently, an individual patient may alternate between AF and AFL or, less commonly, may manifest AF in one atrium and AFL in the other, making the distinction more difficult.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree