The Shared Airway: Management of the Patient with Airway Pathology
Anne C. Kolker
The patient with airway pathology presents a challenge to the surgeon and anesthesiologist. A coordinated approach to evaluating and securing the airway and ultimately treating the underlying problem is critical. Both surgeon and anesthesiologist must have a coherent plan for airway management, including alternatives in cases of difficulty or failure of the initial plan. The patient’s symptoms may range from mild hoarseness to acute dyspnea and agitation, depending on the location, size, and type of lesion. Preoperative evaluation, when possible, helps to define the area and type of the lesion. Appropriate radiologic studies and laboratory tests are valuable. Planning for both the surgical procedure and the conduct of anesthesia is crucial because airway control and the ability to ventilate may be severely limited. Both the surgeon and anesthesiologist require knowledge of various possible approaches and treatments of complex airway problems, particularly in instances in which they must share airway control during manipulation, treatment, or repair of various anatomic problems related to airway patency. Preoperative planning requires a well-organized approach to the various options available for surgery and anesthesia so that the patient receives adequate anesthesia as well as surgical evaluation and treatment.
Causes and Presentation of Patients with Airway Pathology
The underlying airway pathology has many etiologies (Table 23-1). Airway obstruction may be due to intrinsic lesions, including tumors, strictures, webs, malacic segments, and foreign bodies. Extrinsic compression can arise from a range of benign or malignant conditions that result in mediastinal pathology as well as lung or esophageal tumors. Airway disruption can be due to trauma or iatrogenic injury associated with airway manipulation and evaluation. Planned surgery requiring tracheal or bronchial sleeve resection may involve airway disruption during the resection. Prior surgery, tumor, or infection may result in fistulas that evolve over time and are usually small disruptions connecting with a chest cavity or the esophagus. Hemoptysis may be associated with any lesion that connects to the airway.
Intrinsic Lesions
Internal obstruction is most commonly associated with tumors, which can be found at all levels of the trachea and bronchi. These may be primary lung tumors, metastatic lesions, or due to local invasion from the esophagus or mediastinal areas. Strictures can be associated with several types of preexisting lesions. They can arise from pulmonary tuberculosis52 or from other infectious etiologies, including diphtheria, syphilis, and typhoid. Chronic inflammatory conditions can result in tracheal stenosis. Fibrosing mediastinitis and systemic diseases such as Wegener’s granulomatosis or amyloidosis can produce benign strictures. Tracheostomy sites can develop granulation tissue that can mimic a mass or that eventually becomes strictured. Airway burns can develop strictures over time. Prior airway surgery, including tracheal or sleeve resections and lung transplantation, may stricture at an anastomotic site.50 Radiated areas are prone to swelling, followed by strictures. Webs, another cause of airway narrowing, may arise from tracheostomy sites or, in the pediatric population, may be congenital. Relapsing polychondritis and cystic fibrosis can cause airway collapse due to malacic segments. Foreign body aspiration, more often found in the pediatric population or in demented or obtunded patients, presents as an intrinsic obstruction.
Extrinsic Lesions
Extrinsic airway obstruction is most often due to malignancy. Large lung tumors close to the hilum can constrict a main bronchus. Enlarged mediastinal lymph nodes, thyroid, or thymus tissue can compress the anterior trachea.54 Esophageal lesions impinge on the membranous tracheal wall. Airway compression not due to malignancy may stem from tuberculosis, sarcoidosis, or aortic aneurysms. Large mediastinal masses can also compress cardiac chambers and restrict cardiac outflow, in addition to causing airway compression.
Airway Disruption
Loss of airway continuity may present as an emergent problem associated with trauma.28 Iatrogenic injury secondary to intubation,14,44 biopsy, rigid bronchoscopy, or laser treatment
can result in airway disruption. Tracheal or bronchial surgery, including tracheal, carinal, and sleeve resection, creates a temporary loss of airway continuity. Airway fistulas, including bronchopleural or tracheoesophageal fistulas, also constitute a loss of continuity.
can result in airway disruption. Tracheal or bronchial surgery, including tracheal, carinal, and sleeve resection, creates a temporary loss of airway continuity. Airway fistulas, including bronchopleural or tracheoesophageal fistulas, also constitute a loss of continuity.
Table 23-1 Types of Airway Pathology | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Hemoptysis
Hemoptysis is associated with any airway lesion that can erode into a blood vessel. It can also be associated with a friable or vascular endotracheal or endobronchial tumor. Hemoptysis can appear minor, as with blood-tinged sputum, or it can be massive, requiring urgent surgical treatment. If the source of hemoptysis is known or suspected, great care must be taken to avoid any manipulation that will induce bleeding before the patient enters a controlled operating room area.
Preoperative Evaluation
Diagnostic evaluation of patients with obstructive lesions of the airway consists of a detailed history and physical examination, pulmonary function studies, radiographic studies, magnetic resonance imaging (MRI), and bronchoscopy. The indications for each study and the potential benefit derived from the information vary from patient to patient. In addition, the severity and urgency of airway compromise often dictate the diagnostic regimen to be followed. Patients in respiratory distress may acutely decompensate during studies that require respiratory maneuvers or supine positioning. In life-threatening situations where there is a high index of suspicion as to the nature of the airway pathology, diagnosis may consist only of a chest radiograph and bronchoscopy. However, in the patient presenting for elective surgery with symptoms of airway obstruction, a detailed evaluation is generally warranted.
History and Physical Examination
The signs and symptoms produced by airway obstruction are affected by the anatomic location, degree of airway obstruction, and presence of preexisting cardiopulmonary disease. The clinical symptoms generally consist of dyspnea, especially with effort; wheezing, which may present as frank stridor; difficulty clearing secretions; inability to lie flat or lying preferentially on one side; and eventually airway obstruction. These nonspecific symptoms are frequently misdiagnosed, particularly in early stages. It is not uncommon in cases of tracheal tumors to find patients who have been misdiagnosed with asthma that fails traditional treatment modalities and who ultimately come for additional studies. Patients who have extrinsic compression of the airway involving large mediastinal masses must be questioned carefully about their ability to sleep supine. These patients often report that they sleep very little or can sleep only sitting upright. The preferred position is important, because the patient is describing the best position for minimal airway compromise and, therefore, anesthesia induction. If cardiac outflow is known or suspected to be compromised, the preferred position is also critical for anesthesia induction and surgical manipulation.
Patients presenting with loss of airway continuity may be asymptomatic unless there is a large external communication between the airway and another space. A large airway rupture could result in significant dyspnea due to increased work of breathing from loss of tidal ventilation. Generally, with small lacerations, there can be crepitus that develops over time. However, if there is any chance for a ball-valve effect to exist, there should be concern for the possibility of a tension pneumo- thorax or tension pneumomediastinum. Fistulas develop slowly and may cause no significant respiratory symptoms unless there is associated aspiration through the fistula.
Hemoptysis is reported as blood-tinged sputum in most minor cases. The frequency and amount of hemoptysis may herald increased bleeding. Massive or active ongoing hemoptysis can require emergent treatment without benefit of a thorough history, physical examination, or preoperative studies.
Physical examination is essential but may be of limited value in cases of airway obstruction. Chest auscultation frequently reveals diffuse inspiratory and expiratory wheezing that is difficult to differentiate from typical asthma. Audible stridor, which is characterized by both inspiratory and expiratory wheezing, either occurring at rest or provoked with a maximal expiratory effort with an open mouth, is a common finding. Patients with stridor can be dyspneic, using accessory muscles as they struggle to maintain ventilation. Stridor signifies high-grade obstruction (>75%), which requires urgent surgical intervention. Crepitus is the most common presenting sign in instances of airway rupture.
Pulmonary Function Studies
Pulmonary function testing may be useful in helping to define the extent of obstruction. However, patients who present in acute respiratory distress need not be subjected to studies that would further compromise their limited reserve and might precipitate an acute crisis. Although standard spirometry is of limited value in diagnosing obstructive lesions, the use of flow-volume loops has been shown to be very reliable. With standard spirometry, measured airflow during inspiration and expiration may be reduced. Maximal expiratory or inspiratory flow
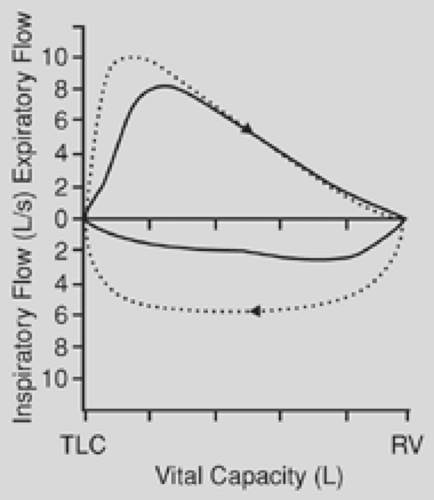
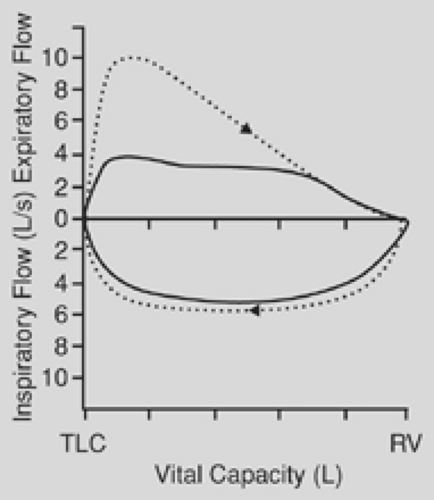
is affected to a far greater degree than is the forced expiratory volume in 1 second (FEV1). The ratio of peak expiratory flow to FEV1 has been used as an index of obstruction. When this ratio is 10:1 or greater, it is suggestive of airway obstruction. The flow-volume loop is the most specific test for the diagnosis of upper airway obstruction. During a forced expiration from total lung capacity, the maximal flow achieved during the first 25% of the vital capacity is dependent on effort alone. In the case of fixed airway obstruction, the peak expiratory flow is markedly reduced, producing a characteristic plateau. With fixed intrathoracic or extrathoracic lesions, the inspiratory flow has the same characteristic plateau (Fig. 23-1). In the case of a variable obstruction—vital capacity in liters, such as that seen with tracheomalacia—the maximal cutoff of inspiratory or expiratory flow depends on the location of the lesion. Extrathoracic or cervical lesions produce a plateau during inspiration, with minimal effect on expiratory flow (Fig. 23-2), whereas intrathoracic lesions that are variable tend to demonstrate alterations in the expiratory flow curve with minimal or no effect on inspiration (Fig. 23-3). In general, circumferential stenoses, such as those produced by cuff lesions, are fixed in origin. Tumors and tracheomalacia frequently produce a variety of intermittent obstructions. It is theoretically possible to estimate the functional impairment of the tracheal lesion by using a restricted orifice in the patient’s mouthpiece as the patient undergoes the flow-volume study. When the limited orifice begins to show additional effect on the flow-volume loop, it can be assumed that the intrinsic lesion has reduced the trachea to that cross-sectional area. In general, airway obstruction must reach 5 to 6 mm in cross-sectional diameter before signs and symptoms become clinically evident. The peak expiratory flow rate decreases to about 80% of normal when the airway diameter is reduced to 10 mm.
is affected to a far greater degree than is the forced expiratory volume in 1 second (FEV1). The ratio of peak expiratory flow to FEV1 has been used as an index of obstruction. When this ratio is 10:1 or greater, it is suggestive of airway obstruction. The flow-volume loop is the most specific test for the diagnosis of upper airway obstruction. During a forced expiration from total lung capacity, the maximal flow achieved during the first 25% of the vital capacity is dependent on effort alone. In the case of fixed airway obstruction, the peak expiratory flow is markedly reduced, producing a characteristic plateau. With fixed intrathoracic or extrathoracic lesions, the inspiratory flow has the same characteristic plateau (Fig. 23-1). In the case of a variable obstruction—vital capacity in liters, such as that seen with tracheomalacia—the maximal cutoff of inspiratory or expiratory flow depends on the location of the lesion. Extrathoracic or cervical lesions produce a plateau during inspiration, with minimal effect on expiratory flow (Fig. 23-2), whereas intrathoracic lesions that are variable tend to demonstrate alterations in the expiratory flow curve with minimal or no effect on inspiration (Fig. 23-3). In general, circumferential stenoses, such as those produced by cuff lesions, are fixed in origin. Tumors and tracheomalacia frequently produce a variety of intermittent obstructions. It is theoretically possible to estimate the functional impairment of the tracheal lesion by using a restricted orifice in the patient’s mouthpiece as the patient undergoes the flow-volume study. When the limited orifice begins to show additional effect on the flow-volume loop, it can be assumed that the intrinsic lesion has reduced the trachea to that cross-sectional area. In general, airway obstruction must reach 5 to 6 mm in cross-sectional diameter before signs and symptoms become clinically evident. The peak expiratory flow rate decreases to about 80% of normal when the airway diameter is reduced to 10 mm.
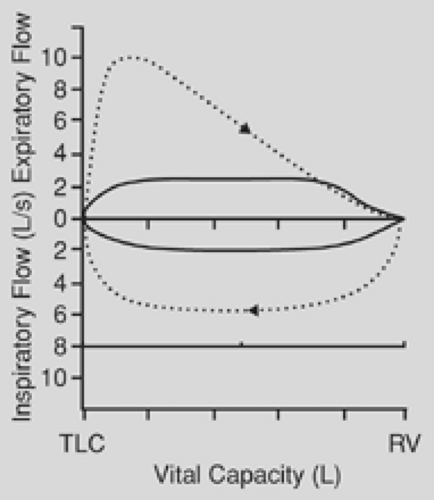 Figure 23-1. Solid line shows a characteristic plateau (both inspiratory and expiratory) of a fixed obstruction. Dotted line shows normal flow-volume loop. |
Pulse oximetry and room-air blood gases give baseline status and indicate the patient’s level of respiratory compensation. Hypoxemia and hypercarbia can result from large airway obstruction of any type and, therefore, should not restrict a patient’s suitability for surgery. Routine preoperative testing, including blood studies and electrocardiography, should also be performed.
Radiologic Studies
Routine and special radiologic studies often demonstrate the location and extent of airway pathology precisely.10,30 Virtual bronchoscopy, a technique that uses a three-dimensional reconstruction of helical computed tomography (CT) data, can be used to navigate through the airways in a fashion similar to bronchoscopy.7,30 Internal renderings allow for visualization of areas beyond a high-grade stenosis and can also be used to examine the airway serially after stent placement. External rendering of images can be used to detect subtle stenosis and tracheomalacia.
Once areas of pathology are identified, tomograms using CT or MRI are useful to define the lesion further. CT scans are used to examine the mediastinum, lung parenchyma, pleura, and chest wall. MRI is better used to delineate the extent of invasive
chest wall tumors, including bone marrow and soft tissue involvement as well as vascular invasion. Some patients who are severely compromised cannot tolerate lying supine for these additional studies. When available, these studies are equally important for the surgeon and anesthesiologist in planning an approach to the airway.
chest wall tumors, including bone marrow and soft tissue involvement as well as vascular invasion. Some patients who are severely compromised cannot tolerate lying supine for these additional studies. When available, these studies are equally important for the surgeon and anesthesiologist in planning an approach to the airway.
Anesthesia Preparation
Monitors
Before any surgical procedure, the patient must be connected to appropriate monitors. Standard monitoring for general anesthesia includes electrocardiography, blood pressure monitors, and pulse oximetry. The pulse oximeter’s tone should be loud enough that all involved staff can easily hear it. A functional intravenous line of any caliber must be established. If cardiac compromise is anticipated, an arterial line should be placed before anesthesia induction.
Airway Evaluation
Careful attention must be paid to evaluating the anatomic configuration and function of the upper airway. Inspection of jaw motion, prominence of upper teeth, adequacy of the oral pharynx, and problems relating to mask fit must be viewed with great concern because prolonged induction with inhalation agents, if required, relies heavily on the ability to maintain an adequate natural airway. In addition, adequate neck extension is required for placement of a rigid bronchoscope.
An airway evaluation should include a discussion between surgeon and anesthesiologist. If any problem is anticipated in securing the airway owing to difficult anatomy, compromised respiratory status, or bleeding, rigid and flexible bronchoscopes should be ready, as well as a skilled intubator. The surgeon should be available before and throughout the induction of anesthesia. Preoperative drug administration is optional depending on the patient’s underlying condition. Antisialagogues may be useful to attempt to diminish secretions associated with repeated airway manipulation. Anxiolytics, usually benzodiazepines, should be used with caution so as not to obtund a patient who is severely compromised.
Surgery and Anesthesia
Bronchoscopy for Intrinsic and Extrinsic Obstruction
Intrinsic Obstruction
Intrinsic lesions can range from distal masses or small areas of stenosis to nearly total airway occlusion from large central airway masses or high-grade stenosis. In cases where airway patency is not considered to be a significant problem, the induction of anesthesia and subsequent airway evaluation may be carried out in a routine fashion. However, patients with significant intrinsic lesions must be approached with concern for possible airway occlusion during anesthesia induction and manipulation of the airway. Intubation with an endotracheal tube may not be possible owing to limited size from stenosis, malacic segment, or tracheal obstruction secondary to a mass.
Extrinsic Obstruction
Extrinsic lesions may cause no airway compromise unless the lesion compresses the trachea or a main bronchus. Patients with airway compression may report an inability to lie flat or a position preference for lying on one side. In cases of airway compression, the intravenous induction of anesthesia may be accompanied by airway collapse. In some instances, extrinsic lesions can compress cardiac chambers. These patients also exhibit a position preference. In these cases, induction of anesthesia may be associated with cardiovascular collapse if the patient’s position is changed. Therefore, the approach to anesthesia induction must allow the patient to remain in the preferred position until the airway is secure and cardiovascular stability is verified.
Evaluation and Induction of Anesthesia
Decisions regarding induction of anesthesia for obstructive airway lesions should follow a logical sequence, as depicted in Figures 23-4 and 23-5. All equipment and experienced personnel must be available and ready as anesthesia induction begins.
Evaluation of a patient with airway pathology includes some type of bronchoscopy for definitive diagnosis. The type of bronchoscopy, flexible or rigid, depends on several factors. The location and size of the lesion may make intubation with an endotracheal tube difficult because of limited airway size or precarious because of the possibility of causing obstruction or bleeding. Both the anesthesiologist and the surgeon must decide whether there will be an adequate airway if intravenous induction is used.
Intravenous induction can be used in cases where airway caliber and integrity are known to be adequate for intubation with an endotracheal tube that is compatible with a flexible bronchoscope. Alternatively, a laryngeal mask airway can be used for initial inspection with a flexible bronchoscope.
When intravenous induction is not considered safe because of factors associated with limited airway size, obstruction, compression, or bleeding, other techniques to secure the airway should be chosen. In the case of high-grade obstruction, intrinsic or extrinsic, rigid bronchoscopy is preferred to both evaluate the area and establish airway control.15,33 If spontaneous ventilation is required owing to intrinsic or extrinsic obstruction, anesthesia can be slowly induced with the patient breathing 100% oxygen and a potent inhalation agent. Patients who are severely compromised—as judged by their tachypnea, dyspnea, agitation, and upright position—need gentle induction with a gradual increase in concentration of the inhaled agent over time so as not to induce coughing or breath-holding. The entire induction may take place in the preferred position in which the patient has arrived. Gentle intravenous induction with dexmedetomidine has also been described.25,47 Pulse oximeter tones must be loud and all attention focused on induction, as there is an ever-present risk of apnea, obstruction, or airway collapse. A rigid bronchoscope and skilled surgeon must be ready for these possibilities. Patients often prefer to be positioned semierect during the initial phase of induction. If airway collapse or cardiovascular compromise is not considered a problem, the patient may be gradually placed supine as anesthesia is deepened. The patient must be given constant coaching to breathe and reassurance that
all is well. The patient’s own airway should remain open without assistance during spontaneous ventilation. Once the patient is adequately anesthetized, as judged by a significant (10%–20%) decrease in blood pressure and heart rate, an attempt at laryngoscopy can be made and the airway secured with either a rigid bronchoscope or an endotracheal tube. In cases where extrinsic compression of the airway or cardiac outflow is anticipated with position change, the patient may have to remain upright until the moment of intubation in order to retain airway patency and blood pressure. If cardiovascular compromise is a known factor, as delineated by preoperative studies, an arterial line should be placed before anesthesia induction if at all possible. If an arterial line cannot be placed, blood pressure must be rapidly checked once the airway is secured so as to ensure that the patient’s position is not a factor in cardiac outflow.
all is well. The patient’s own airway should remain open without assistance during spontaneous ventilation. Once the patient is adequately anesthetized, as judged by a significant (10%–20%) decrease in blood pressure and heart rate, an attempt at laryngoscopy can be made and the airway secured with either a rigid bronchoscope or an endotracheal tube. In cases where extrinsic compression of the airway or cardiac outflow is anticipated with position change, the patient may have to remain upright until the moment of intubation in order to retain airway patency and blood pressure. If cardiovascular compromise is a known factor, as delineated by preoperative studies, an arterial line should be placed before anesthesia induction if at all possible. If an arterial line cannot be placed, blood pressure must be rapidly checked once the airway is secured so as to ensure that the patient’s position is not a factor in cardiac outflow.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree