Fig. 9.1
Posterior view of the pericardial sac, with the anterior surface and heart cut away. One can see that the great vessels of the heart penetrate through the pericardium, which extends up these vessels for several centimeters
9.2 Anatomy
In humans, the 1–3 mm thick fibrous pericardium is commonly described as a flask–shaped bag. The neck of the pericardium (superior aspect) is closed by its extensions surrounding the great cardiac vessels, while the base is attached to the central tendon and to the muscular fibers of the left side of the diaphragm (Fig. 9.2). Much of the pericardium’s diaphragmatic attachment consists of loose fibrous tissue that can be readily separated and/or isolated, but there is a small area over the central tendon where the diaphragm and the pericardium are completely fused.
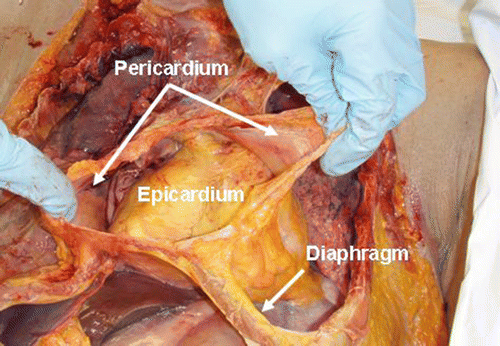

Fig. 9.2
The fibrous pericardium of a fresh human cadaver is opened to expose the epicardium of the heart. Note the attachment of the pericardium to the diaphragm
Examination of the pericardium reveals that it is composed of two interconnected structures: the serous pericardium and the fibrous pericardium. The serous pericardium is one continuous sac with a large infold that contains the heart (Fig. 9.3). An appropriate analogy would be a fist (representing the heart) pushed into the side of a deflated balloon (representing the serous pericardium), therefore enveloped by two individual layers of material. The interior surface of the pericardium is intimately connected to the surface of the heart and is known as the visceral pericardium or the epicardium. The exterior surface of the serous pericardium is known as the parietal pericardium and is fused with the thick lining of the fibrous pericardium. The pericardial space, where the pericardial fluid resides, is bounded on either side by the parietal and visceral pericardium. To the naked eye, however, the visceral pericardium cannot be distinguished from the surface of the heart, and the parietal pericardium cannot be distinguished from the fibrous pericardium. For this reason, the general use of the term pericardium refers to the composite of the parietal and fibrous pericardium, which appears to be a single sac that surrounds the heart. Yet, more accurately, the pericardium should be described as three total layers, with fluid lining between two of them (Fig. 9.3).
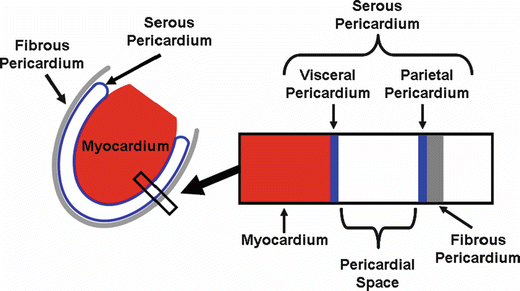

Fig. 9.3
Left: A schematic diagram of the serous and fibrous pericardium with respect to the heart. Right: An expanded cross-section view shows the attachment of two layers of the serous pericardium (visceral and parietal) to the myocardium and fibrous pericardium, respectively
The inferior vena cava enters the pericardium through the central tendon of the diaphragm where there exists a small area of fusion between the pericardium and the central tendon, but it receives no covering from this fibrous layer. Between the left pulmonary artery and subjacent pulmonary vein is a triangular fold of the serous pericardium known as the ligament of the left vena cava (or vestigial fold of Marshall). It is formed by a serous layer over the remnant of the lower part of the left superior vena cava (duct of Cuvier) which regresses during fetal life but remains as a fibrous band stretching from the highest left intercostal vein to the left atrium, where it aligns with a small vein known as the vein of the left atrium (or oblique vein of Marshall), eventually opening into the coronary sinus. The pericardium is also attached to the posterior-sternal surface by superior and inferior sternopericardial ligaments that securely anchor the pericardium and also act to maintain the orientation of the heart inside the thorax.
As previously mentioned, the serous pericardium is a closed sac that lines the fibrous pericardium consisting of a visceral and a parietal portion. The visceral portion that covers the heart and great vessels is commonly referred to as the epicardium and is continuous with the parietal layer that lines the fibrous pericardium. The parietal portion covering the remaining vessels is arranged in the form of two tubes. The aorta and pulmonary artery are enclosed in one tube (the arterial mesocardium), while the superior and inferior venae cavae and the four pulmonary veins are enclosed in the second tube (the venous mesocardium) (see JPG 9.1 in the online supplemental material). There is an attachment to the parietal layer between the two branches, behind the left atrium, commonly referred to as the oblique sinus. There is also a passage between the venous and arterial mesocardia (i.e., between the aorta and pulmonary artery in front and the atria behind) that is termed the transverse sinus. The superior sinus or superior aortic recess extends upward along the right side of the ascending aorta to the origination point of the innominate artery. The superior sinus also joins the transverse sinus behind the aorta, and they are both continually fused until they reach the aortic root. For additional text describing this anatomy, also see Chap. 5.
The arteries of the pericardium are derived from the internal mammary and its musculophrenic branch and also from the descending thoracic aorta. The nerves innervating the pericardium are derived from the vagus and phrenic nerves, as well as the sympathetic trunks.
9.3 Physiology of the Normal Pericardium
9.3.1 Pericardial Fluid
In normal hearts, the pericardium should be considered as only a potential space. It contains 20–60 mL of pericardial fluid, most of which resides in the major pericardial sinuses and the atrioventricular grooves [1]. The fluid is an ultrafiltrate of plasma and therefore has many similarities to plasma in its electrolyte composition; pericardial fluid, however, contains about half the total protein concentration, one-third the triglyceride and cholesterol content, and one-fifth the amount of white blood cells [2]. A more complete comparison of plasma and pericardial fluid composition is shown in Table 9.1.
Table 9.1
Normal plasma composition compared to the pericardial fluid composition of 30 patients undergoing cardiac surgery
Normal plasma range | Pericardial fluid mean value | Mean fluid/serum ratio | |
|---|---|---|---|
Total protein (g/dL) | 6.5–8.2 | 3.3 | 0.6 |
Albumin (g/dL) | 3.6–5.5 | 2.4 | 0.7 |
Glucose (mg/dL) | 70–110 | 133 | 1.0 |
Urea (mg/dL) | 15–45 | 33 | 1.0 |
Calcium (mg/dL) | 8.1–10.4 | 7.3 | 0.9 |
LDH (IU/L) | 100–260 | 398 | 2.4 |
Creatinine (mg/dL) | 0.8–1.2 | 0.9 | 0.9 |
Cholesterol (mg/dL) | 130–240 | 43 | 0.3 |
Triglycerides (mg/dL) | 50–170 | 34 | 0.3 |
White blood cells (K/μL) | 4.0–10.8 | 1.4 | 0.2 |
The details of the formation, clearance, and turnover of pericardial fluid have not yet been fully explained. Yet, it is generally agreed that pericardial fluid is derived from plasma leakage from myocardial capillaries [3], and this filtrate is eventually drained by the lymphatic system. During situations of high pericardial fluid pressure, such as in cardiac tamponade, investigators have found that fluid may pass through the pericardium and enter the pleural space [4]. The turnover time of pericardial fluid in humans has not been established, but in sheep it is observed to be every 5.4 h [5].
As mentioned previously, pericardial fluid distribution is not uniform. The majority of the fluid found in the major sinuses and grooves of the heart and makes up the pericardial reserve volume. The fluid is considered to be well mixed due to the motion of the heart, and agents injected into the pericardial space quickly and evenly disperse throughout [5]. Too much pericardial fluid, either due to disease or an intervention, may cause increased pericardial pressure and compromise cardiac performance, a syndrome called cardiac tamponade. As shown in Fig. 9.4, the pericardial reserve volume acts as a buffer against increasing pressure; once these groves and sinuses have filled, however, the pressure quickly increases with additional fluid volume.
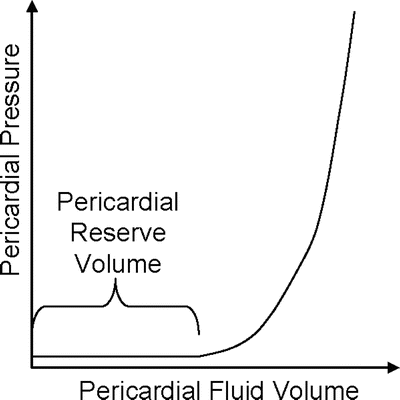

Fig. 9.4
As pericardial fluid volume increases, the pericardial reserve volume is filled. Once the reserve volume is full, pressure within the pericardium rapidly rises and cardiac performance may be compromised. Adapted from Spodick [1]
9.3.2 Mechanical Effects of the Pericardium
The degree to which the pericardium alters heart wall movement(s) varies depending on the ratio of cardiac to pericardial size, loading conditions, and the degree of active and passive filling. Closure of the pericardial sac following open-heart surgery has been proposed to (1): avoid possible postoperative complications, (2) reduce the frequency of ventricular hypertrophy, and/or (3) facilitate future potential reoperations by reducing fibrosis [6]. Furthermore, reported differences in ventricular performance, dependent on the presence of the pericardium, have been observed following cardiac surgery [7, 8].
In general, the presence of a pericardium physically constrains the heart, often resulting in a depressive hemodynamic influence limiting cardiac output by restraining diastolic ventricular filling [9, 10] (see Video 9.1 in the online supplemental material). The physical constraint by the pericardium is translated into direct external mechanical forces that can also alter patterns in myocardial and systemic blood flow [10, 11]. Direct primary and indirect secondary effects are observed as additional forces through the chamber free walls. Because both the left and right side atria and the left and right side ventricles are bound by a common septum, geometrical changes from chamber interaction(s) are dynamic, depending on the different filling rates and ejection rates of each of the four chambers [12, 13]. Thus, it is important to note that chamber-to-chamber interactions through the interventricular septum and by the pericardium further promote direct mechanical chamber interactions [14–16].
The effects of the pericardium on mechanical measures of cardiac performance are generally not evident until ventricular and atrial filling limitations are reached, changing geometrical and mechanical properties through factors such as maximum chamber volume and elasticity. These effects become more evident as the pericardial limitations become extended [17, 18]. With the known force-length dependence of cardiac muscle, variation of chamber volumes through removal of the pericardium will, in turn, alter isometric tensions and therefore directly impact systolic ejection. On the other hand, in specific cases where the restrictive role of the pericardium greatly increases, such as during cardiac tamponade, an increased intrapericardial fluid volume may result in critical restriction by the pericardium that then reduces cardiac performance (Fig. 9.5) [19, 20].
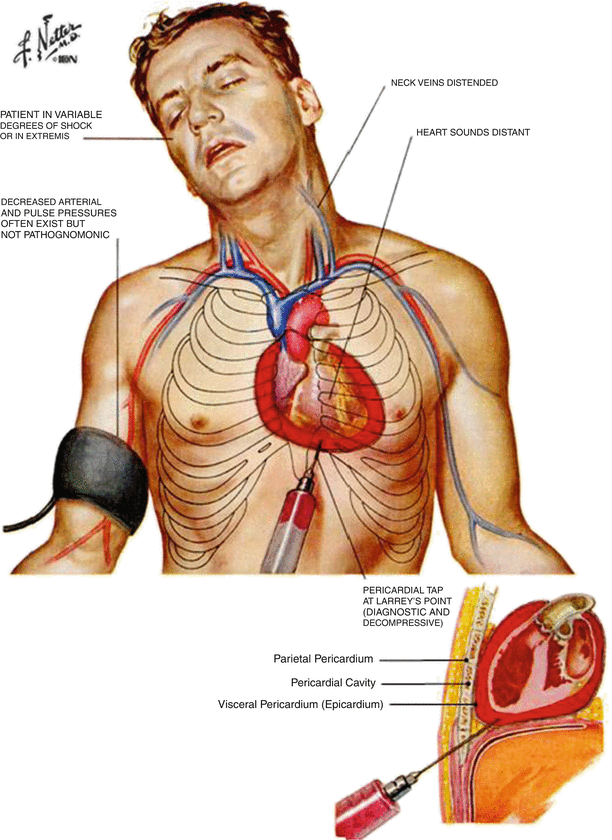

Fig. 9.5
Cardiac tamponade occurs when there is a large accumulation of fluid in the pericardium (top). During tamponade, hemodynamics may be seriously compromised. Distended neck veins, decreased blood pressure, various degrees of shock, and distant heart sounds may all be symptoms of tamponade. In most cases, tamponade is treated by pericardiocentesis or drainage of the sac with a long hypodermic needle (bottom). ©2006 Elsevier Inc. All rights reserved. www.netterimages.com, Frank Netter
It should also be noted that increases in intrathoracic pressure will create an additional interaction between the ventricles, as well as between the heart and lungs in a closed chest. Thus, studying cardiac function in situ (with an opened chest) or in vitro allows for the elimination of these influences of intrathoracic pressure and for more direct identification and quantification of pericardial influences on cardiac performance and ejection [21]. Furthermore, such isolation of these pericardial effects from diastolic filling is an important consideration, since normal ventricular output is dependent on diastolic pressure and independent of the presence of the pericardium [22].
9.4 Pericardial Disorders: Congenital, Pathological, and Iatrogenic
Sir William Osler referred to pericardial disease when he stated that “probably no serious disease is so frequently overlooked by the practitioner” [23]. Many pericardial disorders are asymptomatic and often go unnoticed throughout the patient’s life, but some may be fatal (Fig. 9.6). Pericardial disorders may be classified as congenital, pathological, or iatrogenic.
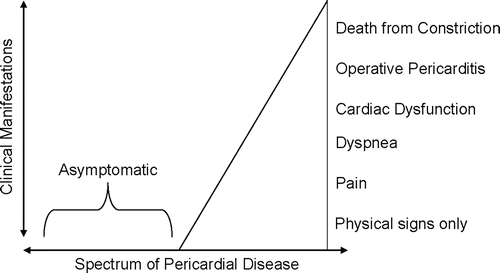

Fig. 9.6
Most pericardial diseases are discovered postmortem, implying that they were asymptomatic throughout the patient’s life. Serious pericardial disease, however, may have many clinical manifestations, as shown in the figure. Adapted from Reddy [22]
Congenital abnormalities of the pericardium are extremely rare. Partial absence of the pericardium may occur, usually exposing the left side of the heart. Complete absence of the pericardium is even less frequent [23]. Cysts may also form during development in, on, or around the pericardium. These usually are not clinically significant and need to be treated only if they become symptomatic [1]. A list of these major congenital abnormalities is found in Table 9.2.
Table 9.2
Major sources of pericardial disorders (congenital, pathological, or iatrogenic)
Congenital | Pathological | Iatrogenic |
|---|---|---|
Primary | Idiopathic pericarditis | Surgical |
Pericardial absence | Due to living agents—infectious, parasitic | Instrument trauma |
Cysts | Vasculitis—connective tissue disease | Cardiac resuscitation |
Teratoma | Immunopathies/hypersensitivity states | Iatrogenic pneumopericardium |
Lymphangioma | Disease of contiguous structures | Drug reactions and complications |
Diverticulum | Disorders of metabolism | Radiation |
Pericardial bands | Trauma—indirect, direct | |
Secondary | Neoplasms—primary, metastatic, multicentric | |
Pericarditis due to maternal lupus | Uncertain pathogenesis | |
Intrapericardial hernia of abdominal organs |
During disease or injury, the pericardium responds with the production of fluid, fibrin, cells, or a combination of the three [23]. The amount of each depends upon the type of disease or injury. Numerous forms of pericarditis can initiate an inflammatory response, including fibrinous pericarditis, fibrous pericarditis, infective pericarditis, and cholesterol pericarditis. Diseases unrelated to the pericardium may also trigger a pericardial response, such as a nearby neoplasm or a myocardial infarction. After a transmural myocardial infarction, for example, patients almost always form adhesions between the necrotic area of the myocardium and the fibrous pericardium [23]. Pericardial effusions, or excess fluid in the pericardium, may occur with disease. Large volumes of lymph, chyle, or blood may accumulate in the pericardial sac. If the accumulation of fluid is significant, this may result in impaired cardiac function. This condition, cardiac tamponade, can be fatal if not treated. A list of several major pathologically induced pericardial disorders is also found in Table 9.2.
Finally, iatrogenic disorders often occur during the treatment of unrelated diseases. During cardiac surgery, the pericardium is often removed (partially or entirely) and is rarely repaired. There has been much debate as to whether closing the pericardium after surgery would be beneficial to the patient. Most surgeons believe that closing the pericardium may acutely compromise postoperative hemodynamics and increase the risk of tamponade. More recent research has shown that there are no clinical benefits, but there may be adverse effects resulting from closure of the pericardium after cardiac surgery [24]. Nevertheless, if the pericardium is left open, the exposed epicardium tends to become very fibrous, complicating future interventions. It should be noted that non-cardiac surgical procedures performed near the heart may also induce trauma to the pericardium and cause an inflammatory response. Furthermore, even nonsurgical interventions may damage the pericardium. For example, resuscitation from cardiac arrest (CPR) may cause a fibrous response. In some patients, irradiation may create an effusion and subsequent tamponade [23]. The pericardium may also adversely react to a number of commonly prescribed drugs such as procainamide, penicillin, doxorubicin, anticoagulants, and/or antithrombotics [1]. The reader is again referred to Table 9.2 for a list of major iatrogenic pericardial disorders.
In general, pericardial disorders can be diagnosed using ECG, echocardiography, radiography, and/or auscultation. Pericardial fluid samples and pericardial tissue biopsies may also aid in complicated diagnoses. Typically, once diagnosed, pericardial disorders are often treatable with a number of drugs and/or interventions [25]. In situations of large pericardial effusions, pericardiocentesis is performed by draining the effusion with a hypodermic needle inserted near the xiphoid process (Fig. 9.5). This is usually done under guidance of echocardiography or fluoroscopy to prevent myocardial puncture, but in emergency situations (such as during acute cardiac tamponade), it may be done without guidance (“blindly”). Chronic effusions or other diseases may necessitate a partial or complete pericardiotomy. This is done by creating an opening into the mediastinum (called a pericardial window) to view and remove portions of the pericardium; visualization of the procedure can be enhanced by a laparoscope or thoracoscope. Balloon pericardiotomy is a recently developed minimally invasive technique that uses a balloon to enlarge a small hole in the pericardium [3].
9.5 Comparative Anatomy of the Pericardium
The pericardium is fixed to the great arteries at the base of the heart and is attached to the sternum and diaphragm in all mammals, although the degree of these attachments to the diaphragm varies between and within species [26, 27]. Specifically, the attachment to the central tendinous aponeurosis of the diaphragm is firm and broad in humans and pigs, the phrenopericardial ligament is the only attachment in dogs, and the caudal portion of the pericardium is attached via the strong sternopericardial ligament in sheep [26, 27] (see Video 9.2 in the online supplemental material).
Although the basic structure of the pericardium is the same, differences exist between various species with respect to both geometry and structure [28–30]. Generally, pericardial wall thickness usually increases with increasing heart and cavity size between the various species [28]. Humans are a notable exception to this rule, having a much thicker pericardium than animals with similar heart sizes [28]. Specifically, the pericardium of human hearts varies in thickness between 1 and 3.5 mm [1], while the average pericardial thickness of various animal species is considerably thinner (ovine hearts, 0.32 ± 0.01 mm; porcine hearts, 0.20 ± 0.01 mm; and canine hearts, 0.19 ± 0.01 mm) [29]). Differences in the relative volume of pericardial fluid also exist. Holt [28] reported that most dogs have between 0.5 and 2.5 mL of pericardial fluid (with some dogs having up to 15 mL), compared to 20–60 mL in adult human cadaver hearts. In the Visible Heart® Lab, we have found that 70–80 kg swine have about 7–8 mL of pericardial fluid. When selecting an appropriate animal model for pericardial access procedures or intrapericardial therapeutics, the significant differences of pericardial thickness, pericardial fluid volume, and pericardial attachments between humans and the various animal models must be considered.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


