Fig. 6.1
Drawing of the coronary arterial circulation in the: (A) dog, (B) pig, and (C) human. Notice the extensive network of coronary collateralization in the dog heart, including many arterial anastomoses. The normal pig and human hearts have significantly less collateralization; each area of myocardium is usually supplied by a single coronary artery. Ao aorta, LAD left anterior descending artery, LCx left circumflex artery, PA pulmonary artery, RCA right coronary artery
6.3 Literature Review of Large Mammalian Comparative Cardiac Anatomy
In general, the hearts of large mammals share many similarities, and yet the size, shape, and position of the hearts in the thoracic cavities can vary considerably between species [10]. Typically, the heart is located in the lower ventral part of the mediastinum in large mammals [11]. Most quadruped mammals tend to have a less pronounced left-sided orientation and a more ventrally tilted long axis of the heart when compared to humans [11] (Fig. 6.2). Additionally, hearts of most quadruped mammals tend to be elongated and have a pointed apex, with the exception of: (1) dogs which tend to have an ovoid heart with a blunt apex [11], (2) sheep which may have a somewhat blunt apex [12], and (3) pigs which have a blunt apex that is oriented medially [12]. Comparatively, human hearts typically have a trapezoidal shape [13] with a blunt apex. However, the apices of normal dog, pig, sheep, and human hearts are all formed entirely by the left ventricles [12–15] (Fig. 6.3).
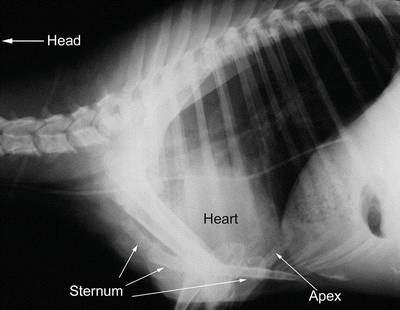
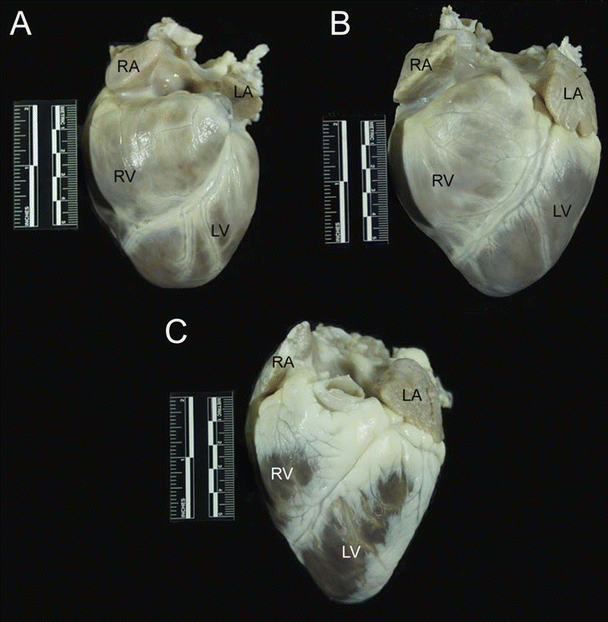

Fig. 6.2
Lateral radiograph of sheep thorax showing orientation of the heart while the animal is standing. The cranial direction is to the left and ventral to the bottom. The apex of the heart is more ventrally tilted (down toward the sternum) than is seen in humans, due to the posture of quadruped mammals. It should be noted, however, that this tilting is limited due to extensive attachments of the pericardium to the sternum and diaphragm

Fig. 6.3
The anterior aspect of the dog (A), pig (B), and sheep (C) hearts. The apex is formed entirely by the left ventricle in these hearts. Also notice the differences in overall morphology of the hearts. The dog heart is much more rounded than the pig and sheep hearts and has a blunt apex. The pig heart has more of a valentine shape with a somewhat blunt apex compared to the sheep heart. The sheep heart is much more conical in shape and has a much more pronounced apex than dog or pig hearts. Also noteworthy is the presence of significant amounts of epicardial fat on the sheep heart, compared with dog and pig hearts. LA left atrium, LV left ventricle, RA right atrium, RV right ventricle
It is important to note that differences exist in the heart weight to body weight ratios reported for large mammals. It is generally accepted that adult sheep and adult pigs have smaller heart weight to body weight ratios than those of adult dogs. More specifically, the adult dog may have as much as twice the heart weight to body weight ratio (6.9 to 7 g/kg) as pigs (2.9 to 2.5 g/kg) or sheep (3.0 to 3.1 g/kg) [16, 17], yet such findings will also likely be breed specific. The normal adult human heart weight to body weight ratio has been reported to be 5 g/kg which, on a comparative note, is similar to that of young pigs (25–30 kg animals) [7].
All large mammalian hearts are enclosed by the pericardium, which creates the pericardial cavity surrounding the heart. The pericardium is fixed to the great arteries at the base of the heart and is attached to the sternum and diaphragm in all mammals, although the degree of these attachments to the diaphragm varies between species [10, 11]. Specifically, the attachment to the central tendinous aponeurosis of the diaphragm is firm and broad in humans and pigs, the phrenopericardial ligament is the only pericardial attachment in dogs, and the caudal portion of the pericardium is attached via the strong sternopericardial ligament in sheep [10, 11].
The pericardium consists of three layers—the serous visceral pericardium (epicardium), the serous parietal pericardium, and the fibrous pericardium. The serous parietal pericardium lines the inner surface of the fibrous pericardium, and the serous visceral pericardium lines the outer surface of the heart. The pericardial cavity is found between the serous layers and contains the pericardial fluid. The pericardium is considered to serve many functions including: (1) preventing dilatation of the heart, (2) protecting the heart from infection and adhesion to surrounding tissues, (3) maintaining the heart in a fixed position in the thorax, and (4) regulating the interrelations between the stroke volumes of the two ventricles [18–20]. However, it should be noted that the pericardium is not essential for survival, since humans with congenital absence of the pericardium and pericardiectomized animals or humans can survive with minimal consequences for many years [18, 21].
Although the basic structure of the pericardium is the same, there are important differences between species [18, 19, 22]. For instance, pericardial wall thickness increases with increasing heart size [18]. Humans are the notable exception to this rule, having a much thicker pericardium than animals with similar heart sizes [18]. Specifically, the pericardium of the human heart varies in thickness between 1 and 3.5 mm [20], while the average thickness of the pericardium of various animal species was found to be considerably thinner (sheep hearts, 0.32 ± 0.01 mm; pig hearts, 0.20 ± 0.01 mm; dog hearts, 0.19 ± 0.01 mm) [19]. Differences in the amount of pericardial fluid are considered to exist as well. Holt reported that most dogs have 0.5–2.5 mL of pericardial fluid with some dogs having up to 15 mL, compared to 20–60 mL in adult human cadaver hearts [18]. For additional information on the pericardium, see Chap. 9.
The normally formed hearts of large mammals consist of four chambers—two thin-walled atria and two thicker walled ventricles. From both anatomical and functional perspectives, the heart is divided into separate right and left halves, with each half containing one atrium and one ventricle. In the fully developed heart with no associated pathologies, deoxygenated blood is contained in the right side of the heart and kept separate from oxygenated blood, which is on the left side of the heart. The normal path of blood flow is similar among all large mammals. Specifically, systemic deoxygenated blood returns to the right atrium via the caudal (inferior in humans) vena cava and the cranial (superior in humans) vena cava, subsequently passing into the right ventricle through the open tricuspid valve. At the same time, oxygenated blood returns from the lungs via the pulmonary veins to the left atrium and then through the open mitral valve to fill the left ventricle. After atrial contraction forces the last of the blood into the ventricles, ventricular contraction ejects blood through the major arteries arising from each ventricle, specifically the pulmonary trunk from the right ventricle and the aorta from the left ventricle. Via the pulmonary arteries, blood travels to the lungs to be oxygenated, whereas aortic blood travels through both the coronary arterial system (to feed the heart) and to the systemic circulation (to oxygenate bodily tissue). For additional discussions of flow patterns and function, see Chaps. 1 and 20.
6.3.1 The Atria
The right and left atria of the adult mammalian heart are separated by the interatrial septum. They are located at what is termed “the base” of the heart. The base receives all of the great vessels and is generally oriented cranially or superiorly, although there are reported differences in orientation among species, which are mostly dependent on the posture of the animal [13, 14, 23]. During fetal development, blood is able to pass directly from the right atrium to the left atrium, effectively bypassing the pulmonary circulation through a hole in the interatrial wall termed the foramen ovale . The foramen ovale has a valve-like flap located on the left atrial side of the interatrial septum, which prevents backflow into the right atrium during left atrial contraction [24]. At the time of birth or soon thereafter, the foramen ovale closes and is marked in the adult heart by a slight depression on the right atrial side of the interatrial wall termed the fossa ovalis [14, 24, 25]; it should be noted that it can remain patent in some individuals, and the rate of patent foramen ovale is comparable in adult humans and domestic swine at approximately 10–30 %. As compared to humans, the fossa ovalis is more posteriorly (caudally) positioned in dogs and sheep [11], but more deep-set and superior in the pig heart [13].
The sinus venosus, a common separate structure in non-mammalian hearts, is incorporated into the right atrium and is marked by the sinoatrial node in large mammals [24, 25]. According to Michaëlsson and Ho [11], all the mammals studied (including dogs, pigs, and sheep) have principally the same atrial architecture including: the sinus venosus, crista terminalis, fossa ovalis, Eustachian valve (valve of the inferior vena cava), and Thebesian valve (valve of the coronary sinus). All large mammalian atria also have an earlike flap called the auricle or appendage [13, 14, 25], although the size and shape of the auricles vary considerably between species [11, 13] (Fig. 6.4). In general, the junction between the right atrium and the right appendage is wide, whereas the junction on the left side is much more narrow [13]. Multiple pectinate muscles are found in both the right and left atrial appendages and on the lateral wall of the right atrium [11, 13, 14] (Figs. 6.5 and 6.6). Commonly, there is one posterior (caudal or inferior) and one anterior (cranial or superior) vena cava, although in some mammals there are two anterior venae cavae [24], and the location of the ostia of the venae cavae entering into the atrium varies [11, 13]. Specifically, the ostia of the inferior and superior vena cavae enter at right (or nearly right) angles in large mammalian animal models while entering the atrium nearly in line in humans [13]. Typically, the extent of the inferior vena cava between the heart and liver is long in domestic animals (>5 cm) and short in humans (1–3 cm) [11]. The coronary sinus ostium is normally located in the posterior wall of the right atrium, but its location can differ slightly between species. Interestingly, the number of pulmonary veins entering the left atrium also varies considerably between species; human hearts typically have four [13] or occasionally five [15], dog hearts have five or six [14], and pig hearts have two primary pulmonary veins [13]. In all large mammalian hearts, the atria are separated from the ventricles by a layer of fibrous tissue called the cardiac skeleton , which serves as an important support for the valves as well as to electrically isolate the atrial myocardium from the ventricular myocardium [23].
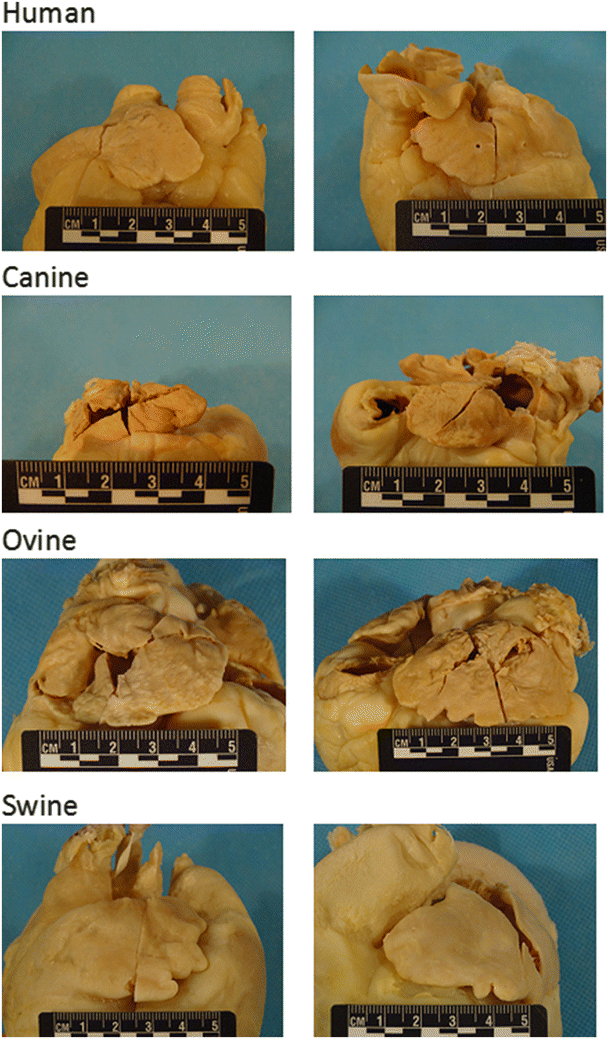
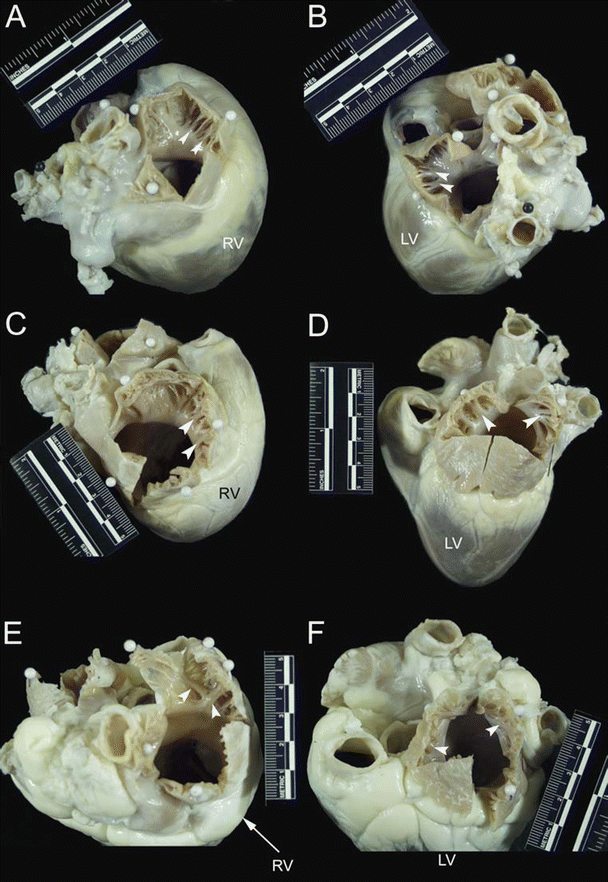
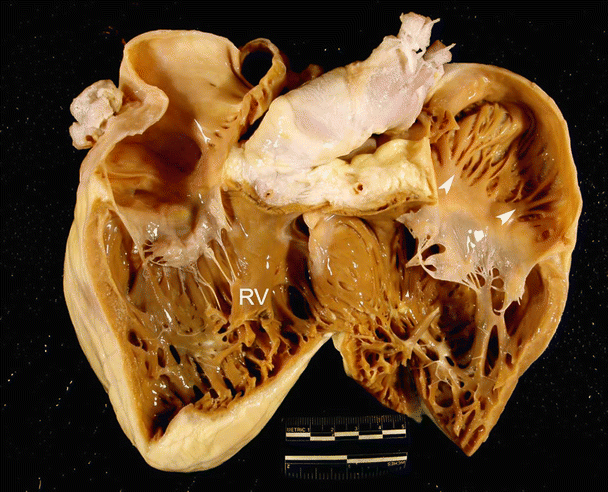

Fig. 6.4
Differences in large mammalian atria. Human: (left) Right atrial appendage is generally triangular in shape and may be larger or smaller than the left atrial appendage; (right) Left atrial appendage is generally tubular in shape. Canine: (left) Right atrial appendage is generally tubular and is larger than or similar in size to the left atrial appendage; (right) left atrial appendage is usually tubular. Ovine: (left) Right atrial appendage is generally half-moon in shape and is larger than the left atrial appendage; (right) left atrial appendage is generally triangular in shape. Swine: (left) Right atrial appendage is usually half-moon in shape and is generally smaller than the left atrial appendage; (right) left atrial appendage is generally triangular in shape. Source: www.vhlab.umn.edu/atlas, Comparative Anatomy Tutorial

Fig. 6.5
The cranial (superior) aspect of dog (A and B), pig (C and D), and sheep (E and F) hearts. Images on the left of the figure (A, C, and E) show opened right atrial appendages, while images on the right (B, D, and F) show opened left atrial appendages. White arrows point to pectinate muscles that line the right and left atrial appendages. Notice that the right and left atrial appendages of the dog heart are tubular in nature. In contrast, the right and left atrial appendages of the pig and sheep heart are more triangular in morphology. LV left ventricle, RV right ventricle

Fig. 6.6
A human heart opened on the inferior and superior aspects of the right ventricle, to show the anterior and posterior walls. White arrows point to pectinate muscles in the right atrial appendage on the anterior aspect. RV right ventricle
6.3.2 The Ventricles
The left and right ventricles of the large mammals used for cardiovascular research essentially contain the same components which are also structurally very similar to those in humans, including: an inlet (inflow) region, an apical region, and an outlet (outflow) region. The ventricles can be considered the major ejection/pumping chambers of the heart, and, as expected, their walls are significantly more muscular in nature than those of the atria. It should also be noted that the left ventricular walls are notably more muscular than those of the right ventricle, due to the fact that the left ventricle must generate enough pressure to overcome the resistance of the systemic circulation, which is much greater than the resistance of the pulmonary circulation (normally more than 4 times greater). The walls of both ventricles near the apex have interanastomosing muscular ridges and columns termed the trabeculae carneae which serve to strengthen the walls and increase the force exerted during contraction [11, 14, 24, 25]. However, large mammalian hearts reportedly do not have the same degree of trabeculation located in the ventricles as normal adult human hearts, and the trabeculations in animal hearts are commonly more coarse than those of human hearts [11, 13] (Figs. 6.7 and 6.8). One can also compare these relative anatomies by carefully studying various prepared plastinated cardiac specimens of large mammalian hearts, including humans. Papillary muscles supporting the atrioventricular valves are found attached to the walls of the ventricles. Similar to human anatomy, in the majority of large mammalian animal hearts, the right ventricle has three papillary muscles, and the left ventricle has two, although variations in individuals and species do occur [11]. It should be noted that, in general, each papillary muscle supplies chordae tendineae to at least 2 leaflets, ensuring redundancy. Both ventricles typically have cross-chamber fibrous or muscular bands, which usually contain Purkinje fibers. Within the right ventricle of most dogs, pigs, and ruminants, a prominent band termed the moderator band is typically present [11]. However, the origin and insertion of the band, as well as the composition of the band, differ notably between species. For example, in the pig heart, the band originates much higher on the septal wall compared to the analogous structure in the human heart [13], and the sheep heart has a similar moderator band as the pig heart (Figs. 6.6, 6.7, 6.8, and 6.9). In the dog heart, a branched or single muscular strand extends across the lumen from the septal wall near, or from the base of, the anterior papillary muscle [14] (Figs. 6.7, 6.8, 6.10, and 6.11). However, Truex and Warshaw [26] did not find any moderator bands in the dog hearts they examined (n = 12), but did observe them in all sheep hearts (n = 12) and all pig hearts (n = 12), compared to 56.8 % of the human hearts they examined (n = 500). Furthermore, they described three subtypes of moderator bands: a free arching band, a partially free arching band, and a completely adherent band. Nevertheless, one must also consider the potential for breed differences in animals and ethnic variability in humans. It is interesting to note that while, in general, anatomical textbooks state there is no specific structure named the moderator band in the left ventricle, left ventricular bands similar to the moderator band of the right ventricle have been described in the literature. For example, Gerlis et al. found left ventricular bands in 48 % of the hearts of children and in 52 % of the adult human hearts studied [27] (Fig. 6.8). They also reported that left ventricular bands were highly prevalent in sheep, dog, and pig hearts [27] (Fig. 6.7).
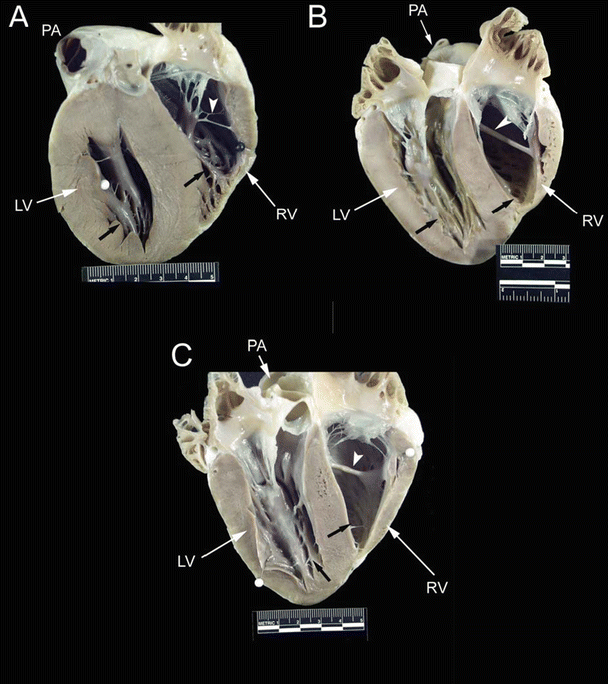
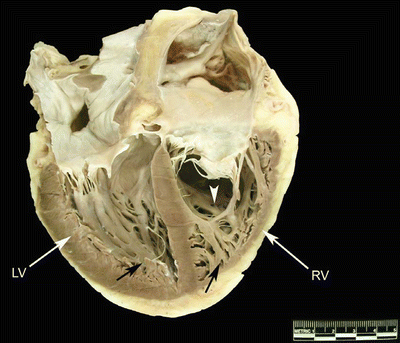

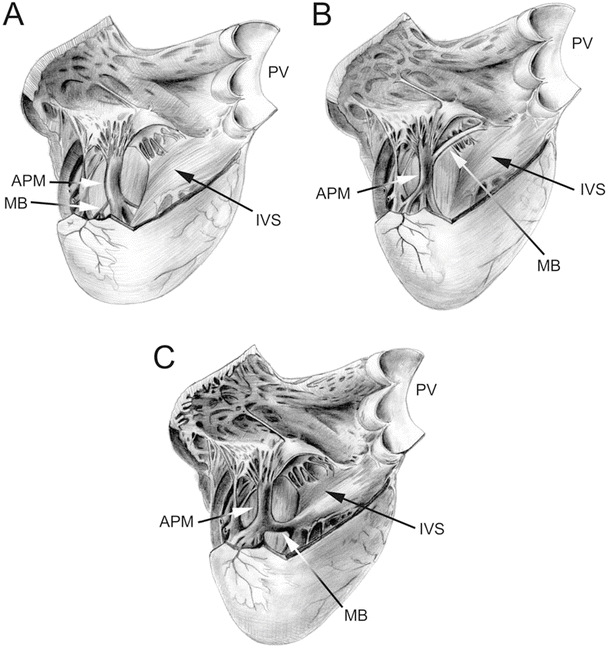
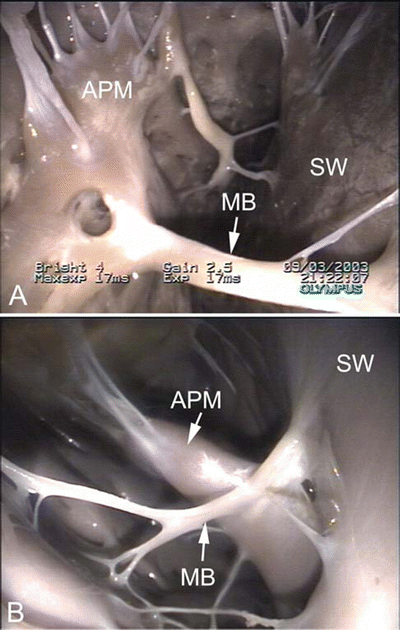

Fig. 6.7
Images showing dog (A), pig (B), and sheep (C) hearts that have been opened along the long axis to show both ventricular cavities . The anterior half of the heart is shown (left ventricle on the left and right ventricle on the right). Black arrows point to ventricular trabeculations which are large and coarse. White arrows point to the moderator band. Notice that a fibrous, branched moderator band extends from the anterior papillary muscle to the free wall in the canine heart. In contrast, a muscular, nonbranched moderator band extends from the septal wall to the anterior papillary muscle in pig and sheep hearts. Additionally, notice the presence of fibrous bands in the left ventricle. LV left ventricle, PA pulmonary artery, RV right ventricle

Fig. 6.8
Image of a human heart opened on the long axis to show both ventricular cavities. The left ventricle is on the left and the right ventricle on the right. Black arrows point to ventricular trabeculations, which are fine and numerous. The white arrow points to the moderator band, which is thick and muscular. It is different in size, shape, and location from the animal hearts shown in Fig. 6.7. LV left ventricle, RV right ventricle

Fig. 6.9
Plastinated hearts of various species. Human: Trabeculae carneae in the apex are notably more numerous and finer than in the hearts of swine, canines, or sheep. Canine: Trabeculae carneae are coarser than those of humans; compared to swine and sheep hearts, the right ventricle has greater trabeculation though the left has similar trabeculation compared to these other animals. Ovine: Trabeculae carneae are noticeably fewer and coarser compared to those in human hearts. Swine: Trabeculae carneae are noticeably fewer and coarser compared to humans. Source: www.vhlab.umn.edu/atlas, Comparative Anatomy Tutorial

Fig. 6.10
Drawing of an opened right ventricular cavity in dog (A), pig and sheep (B), and human (C) hearts. The structure of the moderator band differs greatly between these hearts. In the dog heart, there is a branching fibrous band that runs from the anterior papillary muscle to the free wall of the right ventricle. In the human heart, the moderator band is typically located near the apex and is thick and muscular. In the pig and sheep hearts, the moderator band originates much higher on the interventricular septum and travels to the anterior papillary muscle. It is not as thick as in the human heart but is still muscular in nature. Also, note that the anterior papillary muscle in the dog heart originates on the septal wall, as opposed to originating on the free wall of the human, pig, and sheep hearts. APM anterior papillary muscle, IVS interventricular septum, MB moderator band, PV pulmonary valve

Fig. 6.11
Images showing the moderator band in the right ventricle of an ovine heart (A) and canine heart (B). The moderator band in the sheep is muscular, originating on the septal wall and running to the anterior papillary muscle. In contrast, the moderator band in the canine heart appears fibrous. It originates on the septal wall, runs to the anterior papillary muscle, and continues to the free wall of the right ventricle. APM anterior papillary muscle, MB moderator band, SW septal wall
6.3.3 The Cardiac Valves
Large mammalian hearts have four cardiac valves with principally similar structures and locations. Two atrioventricular valves are located between each atrium and ventricle on both the right and left sides of the heart, and two semilunar valves lie between the ventricles and the major arteries arising from their outflow tracts (the pulmonary artery and aorta). Chordae tendineae connect the fibrous leaflets of both atrioventricular valves to the papillary muscles in each ventricle and serve to keep the valves from prolapsing into the atria during ventricular contraction, thereby preventing backflow of blood into the atria. The semilunar valves—the aortic and pulmonic—do not have attached chordae tendineae and close due to pressure gradients developed across them. See Chap. 34 for more details on valvular structures, function, and defects.
The valve separating the right atrium from the right ventricle is termed the tricuspid valve because it has three major cusps—the anterosuperior (anterior), inferior (posterior), and septal cusps. Typically, there are also three associated papillary muscles in the right ventricle. Interestingly, the commissures between the anterosuperior leaflet and the inferior leaflets can be fused in dog hearts [14], giving the appearance of only two leaflets. Interindividual and interspecies variations in the number of papillary muscles have also been reported [11]. The valve separating the left atrium from the left ventricle is termed the mitral or bicuspid valve because it typically has two cusps, the anterior (aortic) and the posterior (mural). However, according to Netter [15], the human mitral valve actually can be considered to have four cusps, including the two major cusps listed above and two small commissural cusps or scallops; further publications on the mitral valve describe large variations in the number of scallops present in human hearts. Quill et al. studied the relative frequency of such variations in 38 human hearts and showed that the commonly described clefts on the posterior leaflet separating that leaflet into three regions (P1, P2, P3) were present in the majority of hearts; deviant clefts were also present in unexpected locations, such as the anterior leaflet, in some hearts [28]. In large mammalian hearts, two primary leaflets of the mitral valve are always present, but variations in the number of scallops exist and can be quite marked, giving the impression of extra leaflets [11]. A fibrous continuity between the mitral valve and the aortic valve is present in humans and most large mammals, extending from the central fibrous body to the left fibrous trigone [11] (Fig. 6.12). The length of this fibrous continuity, termed the intervalvar septum or membranous septum , varies considerably in length in different animals but notably is completely absent in sheep [29]. There are also differences in the fibrous ring supporting the mitral valve and in the composition of the leaflets of the mitral valve between species. For instance, according to Walmsley, a segment of the ring at the base of the mural cusp is always present in the human heart, but is difficult to distinguish in certain breeds of dogs and is inconspicuous in the sheep heart [29].
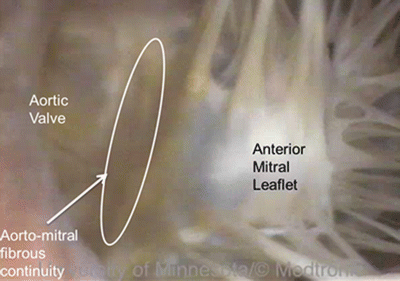

Fig. 6.12
Fibrous continuity between the mitral valve and aortic valve in a human heart . Source: www.vhlab.umn.edu/atlas, Left ventricle/Aortic valve/Visible Heart (functional)/Heart0284-2
Differences in aortic valve anatomy have also been reported in the literature. For example, Sands et al. compared aortic valves of human, pig, calf, and sheep hearts [30], and they reported that interspecies differences in leaflet shape exist, but that all species examined had fairly evenly spaced commissures. Additionally, they found that variations in leaflet thickness existed; in particular, sheep aortic valves were described as especially thin and fragile. They also noted that there was a substantially greater amount of myocardial tissue supporting the right and left coronary leaflet bases in the animal hearts relative to humans [30].
6.3.4 The Coronary System
Mammalian hearts have an intrinsic circulatory system that originates with two main coronary arteries [11] whose ostia are located directly behind the aortic valve cusps. Deoxygenated coronary blood flow returns to the right atrium via the coronary sinus (into which the coronary veins drain) and also to the right atrium, the right and left ventricles [24, 31], and the left atrium [32, 33] by Thebesian veins. According to Michaëlsson and Ho [11], differences in perfusion areas exist between large mammalian species as well as within species (e.g., between breeds); these differences have also been described in humans. Dogs and sheep typically have a left coronary type of supply, such that the majority of the myocardium is supplied via branches arising from the left coronary artery. In contrast, pigs typically have a balanced supply where the myocardium is supplied equally from both right and left coronary arteries [11]. Yet, Crick et al. [13] reported that most of the pig hearts they examined (80 %) possessed right coronary dominance. Additionally, Weaver et al. [34] found that the right coronary artery was dominant in 78 % of the pigs they studied. Most human hearts (approximately 90 %) also display right coronary arterial dominance [35].
Another important aspect of the coronary arterial circulation , one that is of great importance in myocardial ischemia research, is the presence or absence of significant collateralization of the coronary circulation. Normal human hearts tend to have sparse coronary collateral development, which is very similar to that seen in normal pig hearts [34]. In contrast, it is now widely known that extensive coronary collateral networks can be seen in normal dog hearts [5, 36–39]. Furthermore, Schaper et al. [40] found that the coronary collateral network of dogs was almost exclusively located at the epicardial surface, while that of pig hearts, when present, was located subendocardially. They were unable to detect a significant collateral network in the hearts of sheep (Fig. 6.1).
There are three major venous pathways that drain the heart—the coronary sinus, anterior cardiac veins, and Thebesian veins [33, 41]. Drainage from each of these venous systems is present in human hearts as well as in dog, pig, and sheep hearts [13, 14, 24, 33]. While the overall structure of the coronary venous system is similar across species, interindividual variations are common. Nevertheless, there is one notable difference in the coronary venous system between species that warrants mention, that is, the presence of the left azygos vein draining the left thoracic cavity directly into the coronary sinus; a left azygos vein is typically present in both pig [13] and sheep [11] hearts (Fig. 6.13).
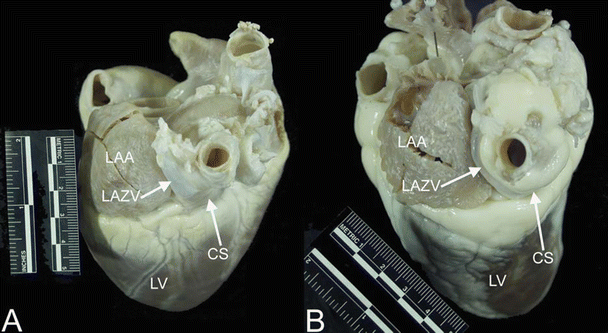

Fig. 6.13
Images showing the left azygos (hemiazygos) vein entering the coronary sinus in the pig (A) and sheep (B) hearts. The left azygos vein drains the thoracic cavity directly into the coronary sinus in these animals, rather than emptying into the superior vena cava via the azygos as seen in dog and human hearts. Notice that it travels between the left atrial appendage and the pulmonary veins; the oblique vein of Marshall (oblique vein of the left atrium) travels this path in human and dog hearts. CS coronary sinus, LAA left atrial appendage, LAZV left azygos vein, LV left ventricle
6.3.5 The Lymphatic System
In addition to an intrinsic circulatory system, large mammalian hearts have an inherent and substantial lymphatic system which serves the same general function of the lymphatic system in the rest of the body. More specifically, the mammalian lymphatic system has been described as follows. Hearts have subepicardial lymphatic capillaries that form continuous plexuses covering the whole of each ventricle [42]. Furthermore, the lymphatic channels are divided into five orders, with the first order draining the capillaries and joining to become the second order and so on, until the lymph is drained from the heart via one large collecting duct of the fifth order. In general, it has been described that dogs, pigs, and humans have extensive subepicardial and subendocardial networks with collecting channels directed toward large ducts in the atrioventricular sulcus that are continuous with the main cardiac lymph duct [43]. Furthermore, it was found that the lymphatic vessels of the normal heart are distributed in the same manner as the coronary arteries and follow them as two main trunks to the base of the heart [44].
6.3.6 The Conduction System
All large mammalian hearts have a very similar conduction system whose main components are the sinoatrial node, atrioventricular node, bundle of His, right and left main bundle branches, and Purkinje fibers. Yet, interspecies variations are well recognized, especially with regard to the finer details of the arrangement of the transitional and compact components of the atrioventricular node [11]. In the mammalian heart, the sinoatrial node is the normal pacemaker [11, 24, 25] and is situated in roughly the same location in all hearts: high on the right atrial wall near the junction of the superior vena cava and the right atrium. Conduction spreads through the atria to the atrioventricular node (which interestingly is unique to both birds and mammals) [25] and then to the bundle of His, which is the normal conducting pathway from the atria to the ventricles, penetrating through the central fibrous body. The right and left main bundle branches emanate from the bundle of His and branch further into the Purkinje fibers which then rapidly spread conduction to the ventricles [11]. The atrioventricular node and bundle of His are typically located subendocardially in the right atrium within a region known as the triangle of Koch , which is delineated by the coronary sinus ostium, the membranous septum, and the septal/posterior commissure of the tricuspid valve (Fig. 6.14). The presence of the os cordis has been noted to be present in the sheep heart, but not in dog, pig, or human hearts. Specifically, it is a small, fully formed bone that lies deep in the atrial septum which, in turn, influences the location and course of the bundle of His in sheep hearts. Other known differences in the atrioventricular conduction system between human, pig, dog, and sheep hearts are illustrated in Table 6.1. For more details on the conduction system, see Chap. 13.
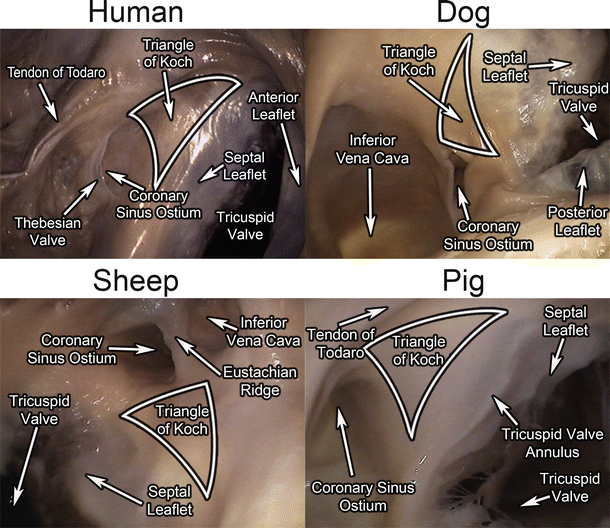

Fig. 6.14
The triangle of Koch in human , dog, sheep, and pig hearts
Location of AV node | AV node and bundle of His junction | Length of bundle of His | Route of bundle of His | |
|---|---|---|---|---|
Human | Located at the base of the atrial septum, anterior to the coronary sinus, and just above the tricuspid valve | End of the AV node and the beginning of bundle of His are nearly impossible to distinguish | Total length of the unbranched portion is 2–3 mm. Penetrating bundle is 0.25–0.75 mm in length. Bundle bifurcates just after emerging from the central fibrous body | Bundle lies just beneath the membranous septum at the crest of the interventricular septum |
Pig | Lies on the right side of the crest of the ventricular septum and is lower on the septum than in humans | No explicit information found | Penetrating bundle is very short in comparison to humans | Climbs to the right side of the summit of the ventricular septum, where it enters the central fibrous body. The bifurcation occurs more proximally than in humans |
Dog | Same as in humans | Consists of internodal tracts of myocardial fibers | Penetrating bundle is 1–1.5 mm long, significantly longer than the human penetrating bundle | His bundle runs forward and downward through the fibrous base of the heart, just beneath the endocardium. There are at least three discrete bundle of His branches of myocardium that join the atrial end of the AV node via a proximal His bundle branch |
Sheep | Located at the base of the atrial septum, anterior to the coronary sinus, just above the tricuspid valve, and at the junction of the middle and posterior one-third of the os cordis | Junction is characterized by fingerlike projections, where the two types of tissue overlap; size and staining qualities of the initial Purkinje cells of the bundle of His make it easy to distinguish between the end of the AV node and the beginning of the bundle of His < div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|