The Pathology of Bronchogenic Carcinoma
There have been profound and fundamental changes in the significance of the histologic classification of bronchogenic carcinoma since the previous edition of this book. The past practice of simply dividing lung tumors into non–small-cell and small-cell lung carcinoma, particularly when evaluating small specimens in patients with advanced disease, was nearly always sufficient for providing the clinically relevant pathologic information that was necessary for treatment purposes. The development of new classes of drugs and targeted therapy for lung cancer has not only prompted an extensive reevaluation of the pathologic classification but has also affected strategies for tissue acquisition and routine processing. Rather than diminishing the traditional role of the pathologist in lung cancer treatment, the advent of what has been termed “personalized” or “precision” medicine has only made pathologic assessment more crucial to patient management.1,2 This chapter focuses on the major histologic subtypes of malignant pulmonary epithelial tumors and includes carcinoid tumors, sarcomatoid carcinoma, and salivary gland tumors. Other unusual tumors, both benign and malignant, are covered in a separate chapter and there is a separate chapter on the genetic and molecular changes in lung cancer. The extremely rapid pace of developments in molecular diagnostics and therapy makes it quite difficult to make enduring summary statements about the prognostic and therapeutic implications for specific histologic subtypes. The overall intent of the chapter is to provide a broad overview of the current histologic classification with its controversies and to provide a deeper understanding of the current issues regarding the preanalytic steps of sampling, processing, and evaluating lung cancer specimens for molecular analysis.
GENERAL CONSIDERATIONS IN HISTOLOGIC CLASSIFICATION AND THE CURRENT CLASSIFICATION OF LUNG TUMORS
Pathologic assessments are continually refined to reflect changes in surgical and medical management, as well as to incorporate an improved understanding of basic tumor biology. Once the diagnosis of malignancy has been made, the pathologic evaluation of lung cancer has traditionally focused on histologic subtyping and, for the minority of patients undergoing surgical resection, determining the extent of disease. Histologic classification is essentially predicated on the assumption that the quantitative predominance of a particular histologic pattern reflects distinctive biologic characteristics. It has been gratifying to note that concurrent developments in other disciplines such as molecular biology have substantiated many aspects of the currently accepted framework for histologic classification. The 2004 World Health Organization (WHO) classification of lung tumors was the first edition to extensively summarize the molecular biology of different tumor subtypes.3 Nevertheless, the main purpose of the 2004 WHO classification was to provide reproducible criteria to pathologists worldwide by using recognizable architectural patterns and individual cellular features that can be appreciated by routine light microscopy and standard hematoxylin- and eosin-stained slides. The use of ancillary techniques, such as immunohistochemistry or molecular biology, is not required in most instances, thereby making the classification accessible to all pathologists for diagnosis and fostering consistency in treatment and research protocols. Although this approach might seem antiquated, it should be noted that the incorporation of data from ancillary studies such as immunohistochemistry or molecular analysis has broad implications, not the least of which is the expense of the laboratory tests, which require additional time, special equipment, technical expertise, and the possibility of exhaustion of small tumor samples that are then no longer available for future testing. Criteria for the interpretation of some of these emerging molecular tests have yet to be well defined and the clinical significance addressed within the confines of prospectively designed, large clinical studies with standardized laboratory analysis.
The 1999 WHO classification introduced significant changes in the classification and nomenclature of malignant epithelial lung tumors from the previous 1982 WHO classification.4 These changes reflected the substantial amount of pathologic observation and translational research that had ensued in the intervening 17 years. The 2004 revision made some minor changes in nomenclature but preserved the overall classification scheme that had been established in 1999. Major changes in the 1999 revision included the introduction of new variants of squamous cell carcinoma, adenocarcinoma, and large-cell carcinoma, as well as new or refined definitions for bronchioloalveolar carcinoma (BAC) and solid adenocarcinoma. The 1999 revisions also incorporated consensus criteria for neuroendocrine tumors and biphasic or pleomorphic tumors. Although it is beyond the scope of this chapter to discuss in detail, it is worth noting that 1999 WHO revisions also added and defined two other preneoplastic processes – atypical adenomatous hyperplasia (AAH) and diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH) – to the previously recognized squamous dysplasia/carcinoma in situ.
Tumor classification and associated generalizations pertaining to tumor type are often made to seem relatively straightforward but, in practice, it is always a challenge for any proposed scheme of histopathologic classification to ensure the reproducible recognition of tumor subtypes. One of the major criticisms of the current WHO classification is that the classification of lung cancer cell types is based on a detailed histologic examination of resection specimens despite the unfortunate fact that the majority of lung cancer patients present with advanced disease and never undergo resection. The 1999 and then the 2004 WHO classifications also had attempted to reconcile the often bewildering heterogeneity of pulmonary adenocarcinomas by introducing the term “mixed” type. In practice this term ended up obscuring major differences between minimally and predominantly invasive adenocarcinomas and the potential prognostic significance of different histologic patterns in adenocarcinomas. The 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society (IASLC/ATS/ERS) International Multidisciplinary Classification of Lung Adenocarcinoma addressed these two major issues by proposing terminology for small biopsies/cytology specimens and by proposing a new classification for adenocarcinomas.5 The overall framework of this classification has been generally accepted and adopted but with some criticism, particularly in regard to the new category of “lepidic predominant adenocarcinoma.”6 Despite these refinements, the degree to which histologic heterogeneity is recognized and tumors classified depends to some extent on sampling techniques, and that may, in turn, have implications for protocol design and data evaluation. The effects of prior therapy must also be considered in subsequent biopsies.
Lung carcinomas are classified according to the best differentiated component and pathologists assign a degree of differentiation to tumors that show differentiation, such as squamous cell carcinoma and adenocarcinoma. This is also known as histologic grading and it is used in tumor pathology as a way of attributing prognostic significance to a specific histologic pattern. It is an assessment as to how much the tumor cells phenotypically resemble a normal cell type, such as a squamous cell or a glandular cell. Histologic grading should not be viewed as a histogenetic determination, that is, that a tumor is derived from a specific cell of origin, such as a squamous cell, or that the tumor cell has “dedifferentiated” from a cell of origin. There are three histologic degrees of differentiation: well differentiated, moderately differentiated, and poorly differentiated. Many lung tumors show a wide variation in differentiation. If a tumor is largely undifferentiated but contains recognizable foci of squamous cell carcinoma or adenocarcinoma, it is classified as a poorly differentiated squamous cell carcinoma or adenocarcinoma, respectively. Some tumors, such as small-cell carcinoma or sarcomatoid carcinoma are, by definition, poorly differentiated. In recent years, the most intense focus has been on developing a standardized histologic grading system for pulmonary adenocarcinomas and these efforts will be summarized in the subsequent section on adenocarcinoma.
Only one study to date has provided data on the interobserver reproducibility for non–small-cell lung carcinomas with routine hematoxylin–eosin slides for the current 2004 WHO classification.7 In this study, which included both community pathologists and expert pulmonary pathologists, overall interobserver reproducibility was only fair (κ = 0.25) when using all 44 diagnostic categories of malignant epithelial tumors, with improvement following consolidation into 10 diagnostic categories (κ = 0.48) or the therapeutically relevant small-cell/non–small-cell distinction (κ = 0.55). Not surprisingly, the study identified better differentiation, in addition to slide quality and pathologist expertise, as important predictive factors for increased interobserver reproducibility. The results help to define a baseline diagnostic agreement that might be used in the validation of other ancillary techniques such as immunohistochemistry or molecular analysis that are proposed to further refine diagnostic criteria.
The histologic subtyping of pulmonary carcinomas on the basis of morphology alone is even more challenging in small samples. With the advent of targeted therapies for non–small-cell carcinoma, further pathologic subclassification beyond the traditional distinction between small-cell carcinoma and non–small-cell carcinoma is now considered critical for selecting appropriate treatment for patients.1 Subsequent sections will address the expanded use of immunohistochemical stains for further histologic subclassification as well as the competing priority of preserving sufficient tumor for molecular analysis.
INVASIVE ADENOCARCINOMA, INCLUDING ADENOCARCINOMA IN SITU AND MINIMALLY INVASIVE ADENOCARCINOMA
Adenocarcinoma is the most common histologic subtype in the United States and in most countries.8 Adenocarcinoma is also the most frequently diagnosed subtype in nonsmokers.9 The classification of pulmonary adenocarcinoma has long been a source of controversy—not only because of its diversity in histologic appearance but also because of a wide spectrum of clinical behavior, radiographic findings, and molecular characteristics associated with the entity. It is worth reviewing a concise history of the revisions in lung adenocarcinoma classification to understand the conceptual framework of 2011 IASLC/ATS/ERS multidisciplinary classification and current unsettled issues. The inherent histologic heterogeneity of many primary pulmonary adenocarcinomas has always made reproducible subclassification difficult and even small adenocarcinomas (<2 cm) can contain more than one histologic pattern.10 This practical problem was addressed by the 1999 WHO revision, which introduced for the first time a mixed subtype to the four previously recognized subtypes of acinar, papillary, bronchioloalveolar, and solid.4 In addition, a strict definition for BAC was adopted in the 1999 revision and retained in the 2004 classification. The definition required that the tumor have a pure lepidic growth pattern without evidence of stromal, vascular, or pleural invasion.3,4 The term “lepidic” means that the proliferation of tumor cells should line the alveolar walls in a uniform manner, using the alveolar walls as a supporting scaffold. It should be noted that prior to the 1999 revision, pathologists widely varied in their assessments. Adenocarcinomas with a minor, usually central, and often more poorly differentiated invasive glandular component along with a peripheral bronchioloalveolar pattern are frequently encountered. In instances in which the peripheral BAC component predominated, it had been a common practice to designate the tumor either as a BAC or an “adenocarcinoma with a BAC pattern/features.” The stricter definition of BAC prevailed in the revisions because of evidence demonstrating that small (<2 cm) solitary tumors with a pure lepidic growth pattern had a 100% 5-year survival.11 Histologically, BACs were divided into two major subtypes, nonmucinous and mucinous, and were grouped together because of a similar “lepidic” growth pattern along alveolar septa. The more common nonmucinous type consists of cuboidal, columnar, or so-called “hobnail” cells with apical nuclei. The mucinous type consists of tall columnar cells with abundant apical pale mucinous cytoplasm and basally located nuclei, sometimes resembling goblet cells. A rare mixed nonmucinous and mucinous or indeterminate subtype was also recognized in the 1999/2004 revisions.
While this classification of BAC made sense from a histologic pattern perspective, it did not really encompass the variability in clinical presentation, radiographic features, or prognosis. Although practice patterns changed somewhat after these revisions, the diagnosis of BAC continued to be used for both small and well-differentiated adenocarcinomas as well as for more advanced stage tumors with obvious diffuse pneumonic spread or lymph node involvement. Moreover, in the years following the publication of the 2004 WHO classification, it became increasingly apparent that the nonmucinous and mucinous cell types have distinctive clinical, radiographic, immunophenotypic, and molecular correlations.12,13 In 1995, Noguchi et al. had suggested that even small foci of invasion in what would otherwise be a pure BAC were associated with a 100% disease-free survival and, following the publication of the 2004 WHO classification, there were a number of other studies that similarly supported quantitation of the lepidic growth pattern and the area of invasion within these “mixed” type adenocarcinomas. These studies focused on a cutoff of 5 mm or less of invasion as a way of defining a “minimally invasive adenocarcinoma” (MIA) with an excellent survival.14,15
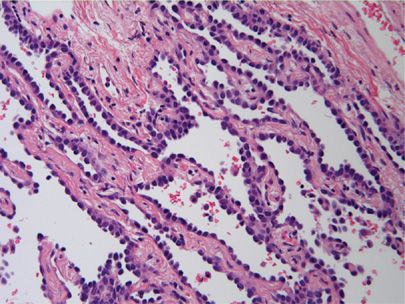
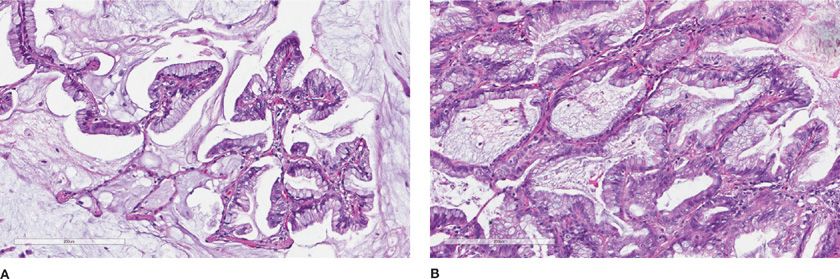
The evidence from these and other studies culminated in the comprehensive 2011 IASLC/ATS/ERS International Multidisciplinary Classification of Lung Adenocarcinoma.5 The 2011 classification completely discards the term BAC in favor of “adenocarcinoma in situ” (AIS). For small (≤3 cm) solitary adenocarcinomas with predominant lepidic growth and small foci of invasion measuring ≤0.5 cm, the term “minimally invasive adenocarcinoma” is recommended. By definition, MIA, can only be applied to a solitary and discrete lesion unless it is clear, in the instance of multiple tumors, that they are synchronous primaries.5 Although the new terminology is a bit unsettling to pathologists and clinicians who have long been used to the entity of BAC, the terms AIS and MIA were favored because they more accurately reflect both the histologic growth pattern and survival statistics. AIS and MIA will typically present as pure ground-glass opacities without a solid component on computed tomography (CT) scans.16 The majority of AIS and MIA cases are nonmucinous, although some cases of mixed nonmucinous and mucinous MIA have been reported (Fig. 111-1).17 Most lesions with a mucinous “BAC” histologic pattern, if carefully examined and sampled, will have invasive foci and, therefore, are classified as “invasive mucinous adenocarcinoma” (Fig. 111-2A,B).
Figure 111-1 Adenocarcinoma in situ, nonmucinous. Uniform proliferation of atypical nonciliated columnar cells with apical nuclei. The tumor cells are growing along the alveolar septa without invasion. (H&E, 200×).
Figure 111-2 Invasive mucinous adenocarcinoma. A. Tall columnar cells with abundant mucinous cytoplasm line the alveolar septa (former mucinous bronchiolalveolar carcinoma). B. Invasive pattern in same tumor. (H&E, 200×).
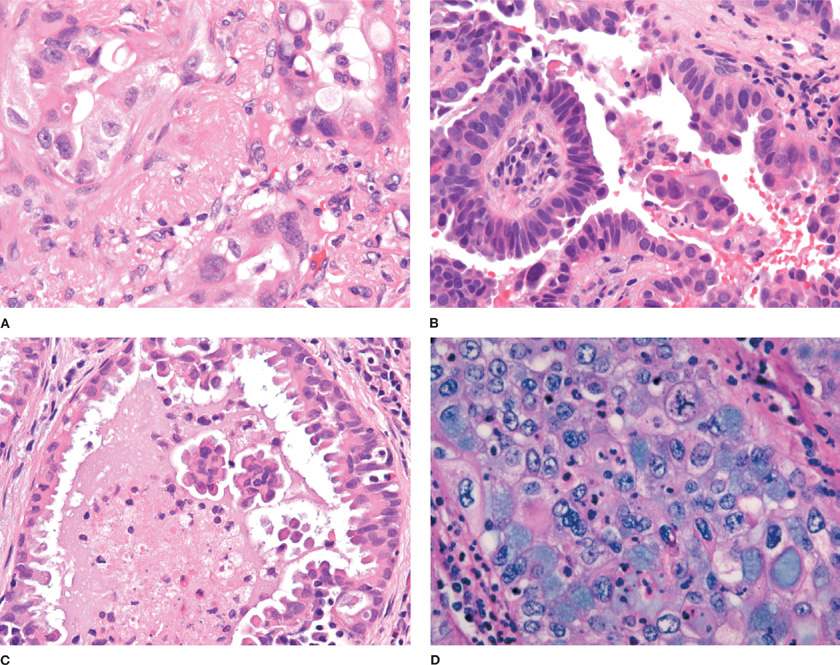
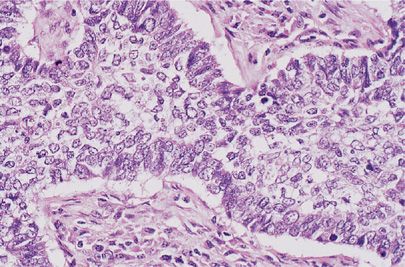
The IASLC/ATS/ERS consensus panel further proposed that invasive adenocarcinomas be characterized by a predominant subtype and recognized five patterns: Lepidic predominant, acinar predominant, papillary predominant, micropapillary predominant, and solid predominant with mucin production. The introduction of a “predominant” pattern was intended as a practical way to evaluate the characteristic heterogeneity of many adenocarcinomas and to allow for better stratification than the “mixed subtype.”5 The recommendation was based on studies in stage I tumors that had suggested that these different histologic patterns had prognostic value in a grading system and that these different patterns can be reproducibly recognized.17–19 Other studies have further validated the prognostic significance of the predominant histologic pattern using this classification system or have evaluated other potential parameters for a grading system including mitotic rate and high thyroid transcription factor-1 (TTF-1) expression within this framework.20–23 Lepidic predominant adenocarcinoma consists mainly of tumor cells growing along alveolar septa (previously referred to as a nonmucinous BAC pattern) but with greater than 5 mm invasion. Acinar predominant adenocarcinoma consists of irregularly contoured but nonetheless recognizable glandular structures and is often associated with a desmoplastic stroma (Fig. 111-3A). Papillary predominant adenocarcinoma consists of malignant cuboidal or columnar cells that line the surface of fibrovascular cores (Fig. 111-3B). Micropapillary predominant adenocarcinoma consists of small papillary clusters of glandular cells growing within airspaces (Fig. 111-3C). The micropapillary pattern was not recognized as a formal histologic subtype in the 2004 WHO classification but has since been recognized in IASLC/ATS/ERS adenocarcinoma classification.5 Solid predominant adenocarcinoma with mucin consists of sheets of tumor cells without an acinar, papillary, or lepidic growth pattern (Fig. 111-3D). The tumor cells have abundant cytoplasmic and mostly vesicular nuclei with prominent nucleoli. Multiple tumor cells have basophilic cytoplasmic vacuoles that suggest intracytoplasmic mucin, which then may be confirmed by a special stain for mucin. The 2004 WHO classification requires that be there five or more mucin-positive cells in at least two high power fields for the diagnosis of solid adenocarcinoma with mucin production. This criterion was adopted because it is not uncommon for squamous cell or large-cell carcinomas to have rare mucin droplets and this is retained in 2011 IASLC/ATS/ERS classification. By definition, given the solid pattern of the tumor, solid adenocarcinomas are poorly differentiated.
Figure 111-3 A. Invasive pulmonary adenocarcinoma, acinar predominant. Small, irregularly shaped glands within a desmoplastic stroma (H&E, 400×). B. Invasive pulmonary adenocarcinoma, papillary predominant. Malignant cells are arranged on the surface of fibrovascular cores (H&E, 400×). C. Invasive pulmonary adenocarcinoma, micropapillary predominant. Small papillary clusters without fibrovascular cores (H&E, 400×). D. Invasive pulmonary adenocarcinoma, solid predominant. Solid nests of cells with mucin (H&E, 400×).
The variants of invasive adenocarcinoma that were defined in the 1999/2004 WHO classifications were reconsidered in the 2011 update and four variants are now recognized: invasive mucinous adenocarcinoma (formerly mucinous BAC), colloid adenocarcinoma, fetal adenocarcinoma (low and high grade), and enteric adenocarcinoma.5 The previously recognized variants of signet-ring adenocarcinoma, and clear cell adenocarcinoma were discarded as discrete entities and are now viewed as cytologic changes that can occur in association with multiple histologic patterns. Although echinoderm microtubule–associated protein-like 4 and anaplastic lymphoma gene fusions (EML4-ALK) may be seen in other histologic subtypes, a significant number of patients with this fusion will demonstrate a solid signet-ring cell pattern (Fig. 111-4).24,25 Enteric adenocarcinoma was added because of its morphologic and immunohistochemical overlap with colorectal adenocarcinomas, thereby necessitating a clinical evaluation to exclude a gastrointestinal primary.5
Figure 111-4 Numerous tumor cells with abundant intracellular mucin (signet-ring cells) that can be seen in association with EML4-ALK fusion. (H&E, 200×).
The reproducibility of histopathologic subtype and invasion in pulmonary adenocarcinomas has been studied.26 In pulmonary adenocarcinomas with classic morphology, there is good reproducibility in identifying a predominant pattern but only fair reproducibility in distinguishing invasion from in situ (wholly lepidic) patterns. Specifically recognizing stromal invasion is still somewhat problematic in practice, even among expert thoracic pathologists. Many adenocarcinomas have areas of fibrosis due to alveolar wall collapse or septal fibrosis with some observers interpreting an individual case as tumor-related stroma with fibroblasts (desmoplastic stroma) and others interpreting as benign fibroelastotic scarring. Histologic criteria for true invasion include single cell infiltration, a fibromyxoid stromal response, high-grade cytology, and a cribriform or acinar growth pattern. This lack of clarity and reproducibility in defining what constitutes invasion in well-differentiated adenocarcinomas makes the subtype of “lepidic predominant adenocarcinoma” the most controversial and there remains some work to be done in terms of refining, standardizing, and improving the recognition of purely in situ disease.6,26
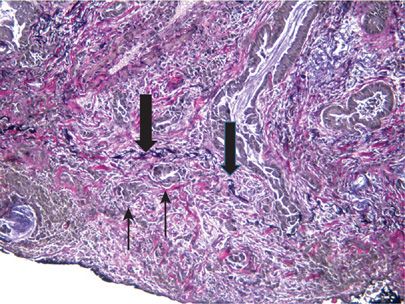
The majority of adenocarcinomas arise in the periphery of the lung and are often associated with parenchymal scarring or puckering of the overlying pleura (Fig. 111-5). It is now a standard recommendation for accurate staging to use elastin stains in the evaluation of visceral pleura invasion in any lesion that approaches the visceral pleura (Fig. 111-6).27,28 Although there are associations of specific subtypes of adenocarcinoma with, for example, EGFR mutations or the EML4-ALK fusion gene, histology is not robust enough to replace molecular analysis in predicting which lung cancers have mutations and are likely to respond to targeted therapy. However, the recognition of these subtypes by the pathologist may play a role in suggesting which molecular tests are most likely to yield positive results for a given cancer and this may be important if there are restrictions due to sample size, test availability, and turnaround time.1
Figure 111-5 Peripheral adenocarcinoma of the lung with pleural puckering.
Figure 111-6 Visceral pleural invasion. Tumor cells (thin black arrow) are present beyond the black elastic layer of the visceral pleura (thick black arrow). (Elastin, 100×).
The histologic heterogeneity of adenocarcinomas is such that the exclusion of metastatic disease or malignant mesothelioma is often an important consideration. As mentioned previously in the enteric variant of adenocarcinoma, the exclusion of a colorectal primary may be important. Other features such as signet-ring cytology raise the possibility of a gastric or appendiceal tumor as well as other primary sites. Mucinous carcinomas, particularly of pancreatic or ovarian origin, may metastasize to the lung and mimic a mucinous adenocarcinoma. All of the classic growth patterns – acinar, papillary, micropapillary, or solid – may be seen in any number of extrapulmonary primaries and clinicoradiologic correlation is always essential. The distinction between peripheral adenocarcinomas with extensive pleural involvement, diffuse carcinomatous involvement of the pleura by metastatic tumor, and malignant mesothelioma may be similarly problematic. Some carcinomas grow in a manner virtually identical to malignant mesothelioma, with extensive pleural involvement and limited parenchymal invasion.29 Clinically, radiographically, and macroscopically, these tumors are indistinguishable from malignant pleural mesothelioma. The histologic appearance may be equally confusing, requiring the use of immunohistochemical stains. As will be discussed further in the general section on immunohistochemistry, there are multiple antibodies that will stain adenocarcinomas of the lung. Many of these antibodies (CEA, MOC31, B72.3, LeuM1, and BerEP4) recognize glycoproteins and are not specific for the lung. Surfactant protein A (SP-A), TTF-1, and Napsin A are the three commercially available markers that can be useful in the diagnosis of lung adenocarcinomas, with the latter two markers (TTF-1 and Napsin A) having higher sensitivity for lung adenocarcinomas.30,31 It is important to recognize that neither TTF-1 nor Napsin A are entirely specific for lung adenocarcinomas and pathologists must still consider within the differential diagnosis a number of extrapulmonary tumors, depending on the clinical circumstances. The combined use of TFF-1 and Napsin A does result in improved sensitivity and specificity for identifying primary pulmonary adenocarcinomas.32
SQUAMOUS CELL CARCINOMA
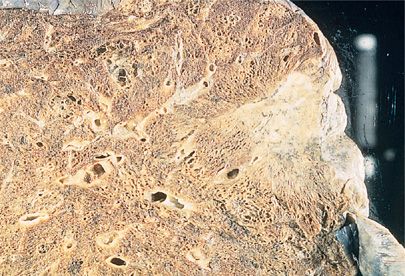
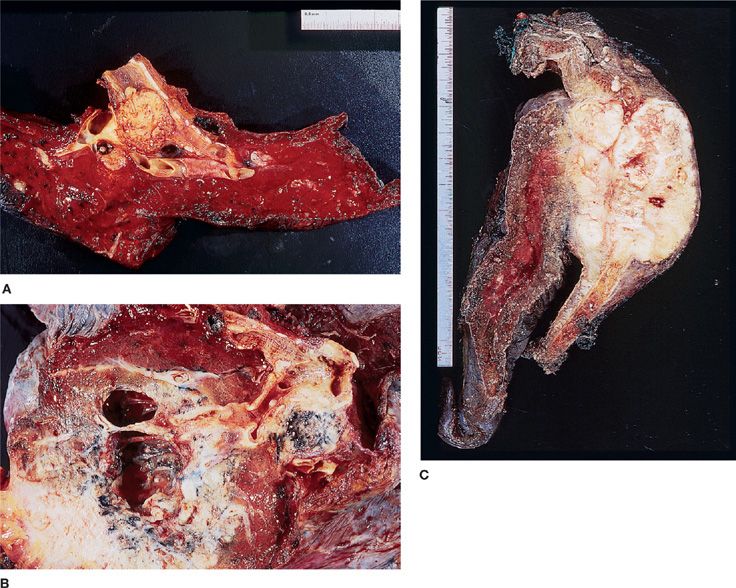
Despite its replacement as the leading lung cancer cell type among both men and women in the United States and in many other countries, squamous cell carcinoma remains an important histologic subtype. Squamous cell carcinoma is seen more commonly in men and is still very strongly correlated with cigarette smoking.33 About two-thirds of squamous cell carcinomas occur centrally, where involvement of a mainstem, lobar, or segmental bronchus may be demonstrated but squamous cell carcinoma may also present as a peripheral mass (Fig. 111-7A,C).34 As would be expected from an endobronchial growth pattern, squamous cell carcinomas frequently are associated with bronchial obstruction and postobstructive pneumonia. Cavitation is seen more frequently in squamous cell carcinoma than in the other histologic subtypes (Fig. 111-7B).3
Figure 111-7 A. Large endobronchial squamous cell carcinoma with atelectasis and obstructive pneumonitis. B. Cavitation within a squamous cell carcinoma. C. Right upper lobectomy with chest wall resection for squamous cell carcinoma.
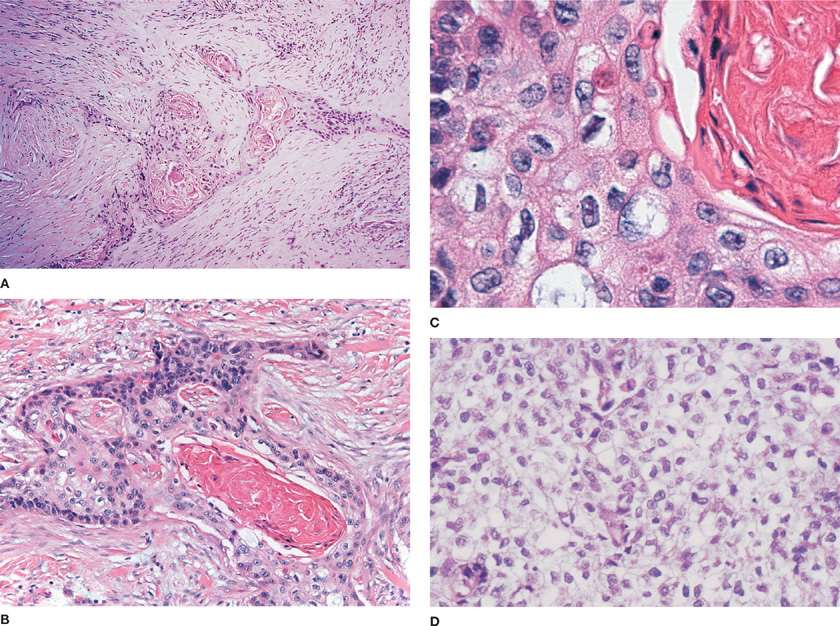
In the 2004 WHO classification, squamous cell carcinoma is defined as a malignant epithelial tumor showing keratinization and/or intercellular bridges. Keratinization may be in the form of squamous pearls or individual cells with dense eosinophilic cytoplasm. Intercellular “bridges” are seen in paraffin sections due to cell shrinkage caused by fixation and correspond to the desmosomal attachments that can be appreciated ultrastructurally (Fig. 111-8B,C). A desmoplastic (i.e., fibrotic) response is often associated with the invasive nests of tumor cells (Fig. 111-8A). As is true of other histologic types, squamous cell carcinomas often show areas of histologic heterogeneity. There are four histologic variants within the 2004 WHO classification of squamous cell carcinoma: papillary, clear cell, small cell, and basaloid patterns.3 On occasion, a tumor may consist entirely of one of these variants, but it is far more common for these patterns to be focal. The papillary variant is characterized by an exophytic growth pattern and papillary cores. The classic tumor cells of a squamous cell carcinoma are large and polygonal with eosinophilic cytoplasm, but in the clear cell variant, as the name suggests, cells with clear cell cytoplasm can be seen (Fig. 111-8D). In the basaloid variant, the nests of tumor cells have prominent peripheral palisading and have less cytoplasm toward the periphery, but the more centrally located cells have more obvious keratinization. In the small-cell variant of squamous cell carcinoma, the tumor cells are relatively smaller and can have granular nuclear chromatin, but there is some chromatin variation with more coarse or vesicular chromatin and prominent nucleoli. A careful search shows cytoplasmic evidence of squamous differentiation in the form of focal keratinization or intracellular bridges. A familiarity with these variant patterns is useful for the practicing pathologist, but at the current time there is no evidence that these variant patterns have any clinical significance. The basaloid and small-cell variants of squamous cell carcinoma can pose a diagnostic dilemma in small, poorly preserved biopsies when their relatively scant cytoplasm mimics small-cell carcinoma. In this instance, a panel of immunohistochemical stains that includes markers of squamous and neuroendocrine differentiation can be useful in interpretation.
Figure 111-8 A. Desmoplastic response with nests of infiltrating squamous cell carcinoma (H&E, 200×). B. Squamous cell carcinoma with keratinization and intracellular bridges (H&E, 200×). C. High power view of keratinization and intercellular bridges (H&E, 400×). D. Tumor cells with clear cytoplasm from a squamous cell carcinoma (H&E, 400×).
Although not invariably demonstrated, it is easiest to identify a trend in tumor progression with the squamous cell histologic subtype. Sampling of a resected specimen may show changes in the adjacent bronchial mucosa ranging from squamous metaplasia to dysplasia to carcinoma in situ. If identified, the presence of an in situ component helps to differentiate a primary squamous cell carcinoma from a metastatic lesion. Aside from a clear-cut transition from in situ to invasive squamous cell carcinoma, there is no other conclusive morphologic means of differentiating a primary pulmonary squamous cell carcinoma from a metastasis. There is a tendency for metastatic tumors from the head and neck to be better differentiated (i.e., show more extensive keratinization) than their primary pulmonary counterparts, but reliably distinguishing a primary squamous cell carcinoma of the lung from other primary sites, particularly from the head and neck, continues to be an ongoing issue. There are studies that have used various molecular techniques to separate a primary squamous cell carcinoma of the lung from a metastasis from the head and neck or addressed these diagnostic issues within the broader context of synchronous or metachronous tumors and metastases.35,36 It is anticipated that additional studies will further validate molecular techniques and facilitate their transition into routine clinical assays that can be used in equivocal clinicopathologic circumstances and when the distinction is critical for treatment or prognosis.
ADENOSQUAMOUS CARCINOMA
This tumor consists of well-defined squamous carcinoma and adenocarcinoma components, with each component comprising at least 10% of the whole tumor.3 The areas of glandular and squamous differentiation may be located in different areas of the tumor or may be intimately admixed. In the past, different criteria had been used for this histologic subtype and these differences in definition, in addition to its low incidence, have made it extremely difficult to compare survival rates with other non–small-cell carcinomas. Prior to the widespread use of immunohistochemistry, the incidence of adenosquamous carcinoma was estimated at 1%.37 It is unclear how the increasing use of immunohistochemistry for histologic subtyping will impact the incidence of this rare tumor. The 2004 WHO criterion of 10% was intended to foster more uniformity in clinical trials and research studies but there is still only a limited amount of data available on this histologic subtype. There are studies that have reported a worse prognosis with adenosquamous carcinoma when compared to other non–small-cell carcinomas of the lung.38–40 The question as to whether adenosquamous carcinomas are a simple mix of adenocarcinoma and squamous cell carcinoma or whether they are more complex at a molecular level is an interesting one that may have a significant impact on targeted therapy selection. There is a limited amount of data that suggests that these tumors are not simple mixtures of two histologic components but rather behave as their own unique entity.41,42
LARGE-CELL CARCINOMA
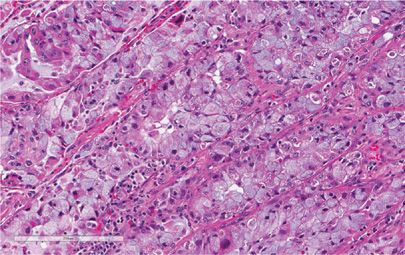
Large-cell carcinomas account for a little less than 10% of all lung cancers.37 Large-cell undifferentiated carcinoma is defined in the 2004 WHO classification as “an undifferentiated malignant epithelial tumor that lacks the cytologic features of small-cell carcinoma and glandular or squamous differentiation.”3 The tumor cells typically have large nuclei, prominent nucleoli, and a moderate amount of cytoplasm (Fig. 111-9). As is evident from this description, this tumor is defined more by what it is not than what it is. For practical purposes, it is a diagnosis of exclusion, and the diagnosis of large-cell carcinoma requires morphologic examination of the resected tumor to rule out areas of squamous or glandular differentiation. The WHO criteria for large-cell carcinoma are based on conventional microscopy, the occasional use of mucin stains, and the required use of immunohistochemistry for one variant (large-cell neuroendocrine carcinoma). Melanoma, malignant large-cell lymphomas, and epithelioid sarcomas also can mimic large-cell carcinoma, typically requiring the use of immunohistochemistry to exclude these diagnoses. The major change in the 1999/2004 WHO revisions was to expand the number of variants included within the category of large-cell carcinoma and transfer others, namely giant cell carcinoma and spindle cell carcinoma, into the sarcomatoid carcinoma category. The large-cell carcinoma variants now include large-cell neuroendocrine carcinoma and combined large-cell neuroendocrine carcinoma (to be covered in the section on neuroendocrine tumors) in addition to basaloid carcinoma, lymphoepithelioma-like carcinoma, clear cell carcinoma, and large-cell carcinoma with rhabdoid phenotype (discussed below).
Figure 111-9 Large-cell carcinoma of the lung. There is no obvious squamous differentiation in the form of keratinization or intercellular bridges and a mucin stain was negative (H&E, 400×).
Despite this definition of large-cell carcinoma based solely on morphology, it has long been known and generally acknowledged that the majority of these tumors represent either very poorly differentiated adenocarcinomas or squamous cell carcinomas. By electron microscopy, many large-cell carcinomas show focal ultrastructural features consistent with adenocarcinoma or a poorly differentiated squamous cell carcinoma.43 Similar subsets within large-cell carcinoma can be defined using the same immunohistochemical stains that are routinely employed to distinguish between adenocarcinoma and squamous cell carcinoma and the distribution of targetable mutations can be generally correlated with the immunophenotyping profile.44 The extent to which morphologically identifiable large-cell carcinomas with immunohistochemical marker profiles of adenocarcinoma or squamous cell carcinoma should be reclassified for prognostic and therapeutic purposes has not been resolved. It is nevertheless clear that there are therapeutically relevant genetic alterations within these “undifferentiated” carcinomas that have practical implications for predictive molecular testing strategies and individualized therapy.
 OTHER VARIANTS OF LARGE-CELL CARCINOMA
OTHER VARIANTS OF LARGE-CELL CARCINOMA
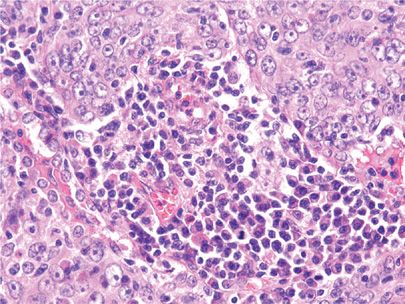
In the 1999/2004 WHO revisions, basaloid carcinoma of the lung is considered to be a variant of large-cell carcinoma. The basaloid histologic features in lung carcinomas are similar to those also seen in other extrapulmonary sites such as the head and neck or cervix. Basaloid cells are typically described as relatively small monomorphic cuboidal to fusiform cells with moderately hyperchromatic nuclei, finely granular chromatin, absent or focal nucleoli, scant cytoplasm, and a high mitotic rate. Intercellular bridges and/or individual cell keratinization should not be present. The tumor cells are usually arranged in lobular, trabecular, or palisading growth patterns (Fig. 111-10). Since these types of histologic patterns can also be seen in neuroendocrine tumors, immunohistochemical stains for neuroendocrine markers should be negative or extremely focal. If the basaloid component is less than 50% and combined with a squamous cell carcinoma, the tumor is classified as squamous cell carcinoma (basaloid variant). One large series of basaloid carcinomas that included both tumors as a variant of large-cell carcinoma and those associated with a squamous cell component suggested that the basaloid pattern itself confers a poor prognosis.45
Figure 111-10 Basaloid carcinoma of the lung. The tumor cells are relatively small with hyperchromatic nuclei and scant cytoplasm. Note the tendency of the tumor cells to palisade at the periphery of the tumor nest (H&E, 400×).
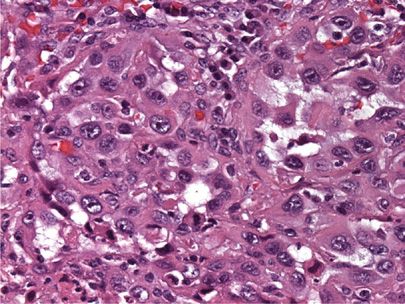
Lymphoepithelioma-like carcinomas are extremely rare in the Western population but are more frequently reported in Chinese patients.46 This rare pulmonary tumor was first reported in the lung in 1987 and the morphologic features are identical to undifferentiated nasopharyngeal carcinoma of the head and neck.47 Large malignant cells with prominent nucleoli are arranged in nests within a lymphoid-rich stroma (Fig. 111-11). In Eastern Asian patients, Epstein–Barr virus EBER-1 RNA has been demonstrated to be present in the large tumor cells.46
Figure 111-11 Lymphoepithelioma-like carcinoma. Large malignant cells with prominent nucleoli are arranged in nests within a lymphoid-rich stroma (H&E, 400×).
The clear cell carcinoma variant consists of a pure clear cell carcinoma without evidence of squamous or glandular differentiation. The tumor consists of large polygonal cells with clear or foamy cytoplasm.3 In addition to other primary lung tumors, the differential diagnosis of a large-cell carcinoma with prominent clear cells should include tumors from other primary sites such as the kidney.
Large-cell carcinoma with rhabdoid phenotype
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree