Chapter 39 The Parathyroid Glands
History
Advances in parathyroid surgery have been colorful and international. Although the Swedish medical student Ivar Sandstrom is credited with first describing the “glandularae parathyrtreoidae” in 1880,1 Sir Richard Owen made the original description in 1850.2 Understanding of parathyroid function predated appreciation of the glands themselves; tetany was described in 1879 in a patient who underwent thyroidectomy (and incidental parathyroidectomy), and the connection between the parathyroids and tetany was identified in 1891.3 Famous patients with HPT include Albert Gahne, a Viennese tram car conductor who underwent two separate parathyroid resections in the 1920s by Felix Mandl for what was most likely parathyroid carcinoma,4 and Captain Charles Martell, a Merchant Marine captain who underwent seven operations and was eventually found to have a mediastinal parathyroid adenoma.5 Both men succumbed to their disease and the consequences of its treatment.
The relationship between chronic renal disease and HPT was first suggested by Albright and colleagues in 1934.6 Castleman and Mallory7 described the pathologic finding of parathyroid hyperplasia of chief cells with marked gland enlargement. Stanbury and associates8 described renal rickets, azotemic osteomalacia, and azotemic HPT and also performed the first subtotal parathyroidectomy as definitive therapy for renal osteitis fibrosa. Rasmussen and Craig9 and, independently, Berson and coworkers,10 extracted a stable homogeneous parathyroid polypeptide and demonstrated that hypercalcemia and phosphaturic properties reside in parathyroid hormone (PTH). Berson and Yalow won the Nobel prize in 1977 for developing an immunoassay for the measurement of PTH, and Reiss and Canterbury11 developed an assay to measure the C-terminal and later the midmolecule portion of PTH.
Calcium Physiology
Calcium exists in extracellular plasma in a free ionized state, as well as bound to other molecules. So-called normal plasma levels of total calcium vary among laboratories, but the range of (bound and unbound) calcium is usually between 8.5 and 10.2 mg/dL (2.2 and 2.5 mmol/liter). The biologically inert bound fraction (55% of the total) binds to proteins. Changes in albumin alter total calcium levels significantly because most protein-bound calcium associates with albumin (80%). A small percentage of calcium is associated with other proteins, such as β-globulins, or with nonprotein molecules, such as phosphate and citrate. Mathematical formulas correcting for disparate albumin levels (e.g., corrected calcium = 0.8-mg/dL decrease for every 1.0-mg/dL decrease in albumin; [total calcium + 0.025] × [40 − albumin]) are notoriously inaccurate. Consequently, ionized calcium levels are measured when required. Forty-five percent of the total calcium is biologically active and exists in the ionized form, with a normal level of 4.5 to 5.0 mg/dL. Ionized calcium levels are inversely affected by the pH of blood; a 1-unit rise in pH will decrease the ionized calcium level by 0.36 mmol/liter.12 Accordingly, patients who are hypocalcemic and hyperventilate can enhance their hypocalcemic symptoms, including perioral paresthesia, tingling in the fingers and toes, muscle cramping, and seizures.
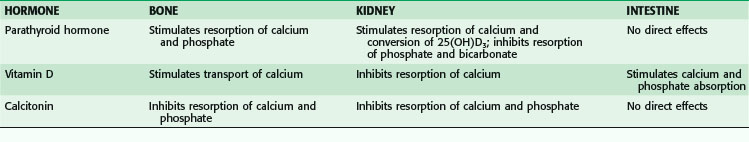
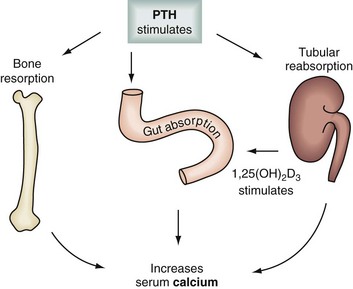
Levels of calcium are highly modulated through a delicate interplay among PTH, calcitonin, and vitamin D acting on target organs such as bone, kidney, and the gastrointestinal (GI) tract (Table 39-1; Fig. 39-1). Chief cells in the parathyroid glands secrete PTH, an 84–amino acid protein, whenever serum calcium levels fall. PTH binds to its peripheral receptors and stimulates osteoclasts to increase bone resorption, to the kidney to increase calcium resorption and renal production of 1,25-dihydroxyvitamin D3 (1,25[OH]2D3), and to the intestine to increase absorption of calcium and phosphate. Together, these processes raise the serum calcium level. The recently cloned calcium-sensing receptors (CaSRs) in the parathyroid glands detect changes in calcium levels, which results in a negative feedback loop that decreases PTH production.
Anatomy
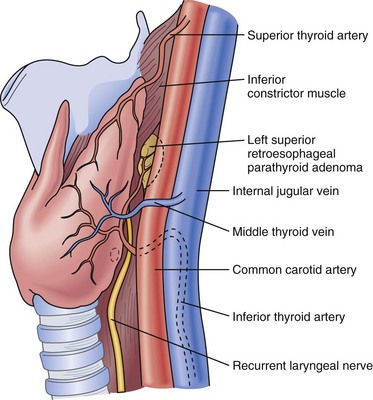
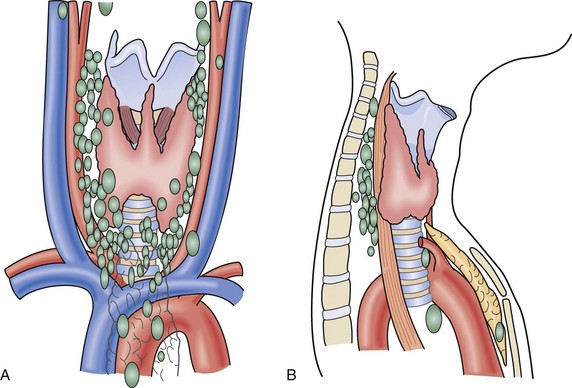
There are usually four parathyroid glands, which lie on the posterior surface of the thyroid. The superior glands are normally located on the posteromedial aspect of the thyroid near the tracheoesophageal groove, whereas the inferior parathyroids are more widely distributed in the region below the inferior thyroid artery (Fig. 39-2). Common sites for ectopic parathyroids are the thyrothymic ligament, superior thyroid poles, tracheoesophageal groove, retroesophageal space, and carotid sheath (Fig. 39-3).13 The percentage of individuals with supernumerary glands varies in published series from 2.5% to 22%.14 The average weight of a normal parathyroid gland is 35 to 40 mg; in adults, its color turns to yellow as the fat content increases. The inferior parathyroids originate from the third branchial pouch, whereas the superior parathyroids descend from the fourth branchial pouch. The superior and inferior parathyroid glands receive their blood supply from the inferior thyroid artery in 80% of cases. Each parathyroid gland generally receives a single end-artery blood supply that is vulnerable to injury during surgical manipulation. The glands are made up of chief and oxyphil cells, as well as fibrovascular stroma and adipose tissue.
Diagnosis and Clinical Features
Primary HPT is the third most common endocrine disorder, after diabetes mellitus and thyroid disease. Middle-aged and older women are most commonly affected by the disease. It is characterized by hypersecretion of PTH, leading to hypercalcemia. Box 39-1 lists the differential diagnosis for hypercalcemia. The diagnosis is made by demonstrating elevated serum calcium and intact PTH (iPTH) levels and normal or increased urinary calcium levels in the setting of normal renal function. In up to 15% of patients, serum PTH levels fall within the upper normal range, but these levels are inappropriate relative to the elevated serum calcium levels. A 24-hour urine collection can help exclude the diagnosis of benign familial hypocalciuric hypercalcemia (BFHH), which results in mild increases in blood calcium and iPTH levels but low urinary calcium. Whereas the calcium-to-creatinine (Ca/Cr) clearance ratio is typically less than 0.01 in patients with BFHH, the ratio is usually more than 0.02 in primary HPT. BFHH is a generally benign condition transmitted in an autosomal dominant fashion that cannot be corrected by parathyroidectomy.
Box 39-1 Adapted from Mulder JE, Bilezikian JP: Acute management of hypercalcemia. In Bilezikian JP, Marcus R, Levine MA (eds): The parathyroids, ed 2, San Diego, Calif, 2001, Academic Press, p 730.
Differential Diagnosis of Hypercalcemia*
The clinical entity termed normocalcemic primary hyperparathyroidism has recently been described.15 It appears to be an early form of primary HPT in which patients have serum calcium levels in the high-normal range associated with an elevated serum PTH level. When these patients are symptomatic, surgical intervention is appropriate.
Hypoparathyroidism
Hypoparathyroidism is an endocrine disorder in which hypocalcemia and hyperphosphatemia are the result of a deficiency in PTH secretion or action. The most common cause of hypoparathyroidism is damage to the parathyroid glands during thyroidectomy, but it can also occur after parathyroid exploration (see later, “Postoperative Complications”). The signs and symptoms of hypocalcemia are caused by neuromuscular excitability from reduced plasma ionized calcium. Early manifestations include perioral numbness and tingling in the fingers. Anxiety or confusion can follow, and it is important for the surgical team to reassure patients early to reduce psychiatric and neurocognitive symptoms. Anxiety often results in hyperventilation, which can then lead to respiratory alkalosis and a further reduction in the serum calcium level. Tetany, marked by carpopedal spasm, convulsions, or laryngospasm (or any combination of the three), may follow and can be fatal. Physical examination includes testing for a Chvostek sign, which is contraction of the facial muscles after tapping on the facial nerve anterior to the ear. Approximately 15% of normal individuals have a positive Chvostek sign, however.
Hyperparathyroidism
Primary Hyperparathyroidism
Effects of Surgery
Even though a National Institutes of Health (NIH) consensus conference was conducted in 1990, another workshop was held in 2002, and an international workshop was held in 2008 on the management of asymptomatic primary HPT, there is still no consensus among endocrinologists and endocrine surgeons about whether to administer nonoperative medical therapy and monitor patients or to refer them for early parathyroidectomy. Criteria for surgery have been established according to the best evidence to date (Box 39-2).16 To some extent, the role of parathyroidectomy in asymptomatic patients with mild to moderate hypercalcemia is debated because the natural history of the disease is still not well understood. Overall, rapid increases in the serum calcium level, progression of symptoms of complications, or both are uncommon in patients with borderline hypercalcemia. However, because of the long-term deleterious effects on bone mineralization, the pendulum has shifted to surgical intervention.17
Box 39-2 Adapted from Bilezikian JP, Khan AA, Potts JT Jr: Third International Workshop on the Management of Asymptomatic Primary Hyperthyroidism: Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the third international workshop. J Clin Endocrinol Metab 94:335–339, 2009.
Criteria for Surgical Referral
Serum calcium concentration >1 mg/dL (>0.25 mM/liter) above the upper limits of normal
All individuals with primary hyperparathyroidism and <50 yr
Patients for whom medical surveillance is undesirable or impossible
Neuromuscular symptoms of primary HPT vary in expression and response to parathyroidectomy among series. However, proximal muscle weakness detected by examination of the isokinetic strength of knee extension and flexion appears to have a higher prevalence and good response to parathyroidectomy, as does respiratory muscle capacity. Psychiatric symptoms such as mental dullness, confusion, and depression are a focus of ongoing investigations. In a study by Roman and coworkers,18 55 patients with primary HPT and benign euthyroid thyroid disease referred for surgery were evaluated preoperatively and postoperatively with validated psychometric and neurocognitive instruments. Patients with primary HPT reported more symptoms of depression preoperatively that improved postoperatively. Preoperatively, patients with primary HPT also showed greater delays in spatial learning. All subjects learned across the neurocognitive trials, but primary HPT patients were more delayed. After surgery, primary HPT patients improved and functioned at a level equivalent to that of patients with thyroid disease. The authors concluded that primary HPT may be associated with a deficit in spatial learning and processing that improves after parathyroidectomy. Several other studies have supported the possibility that patients with primary HPT may exhibit neurocognitive changes, and that these traits may show some improvement after parathyroidectomy.
Significant increases in bone mineral density in the lumbar spine and hip occur after parathyroidectomy, and these improvements are durable. Changes in bone remodeling and density are apparent within 6 months of surgery. Cohort studies have shown that fracture risk declines after parathyroidectomy. No effect of successful surgery has been noted on hypertension or renal impairment. Urinary calcium excretion and the incidence of nephrolithiasis are reduced by surgery. Currently, there are still no convincing data proving that surgical cure increases life expectancy. A Swedish case-control study conducted retrospectively demonstrated that 23 patients who underwent parathyroidectomy had a hazard ratio for death of 0.89 in comparison to matched controls in the normal population, but the numbers were too small to achieve statistical significance.19
Noninvasive Preoperative Localization
A major advance has been improvements in imaging techniques. This has led to the development of more localized surgery, with the opportunity for short operation times, the use of local or regional anesthesia, and limited or no hospital stay. There is now consensus—as marked by the recommendation of the 2002 NIH workshop and the 2008 international workshop—that preoperative localization is imperative before primary exploration if unilateral exploration is desired. Localization continues to be essential before all remedial parathyroidectomies (Table 39-2).
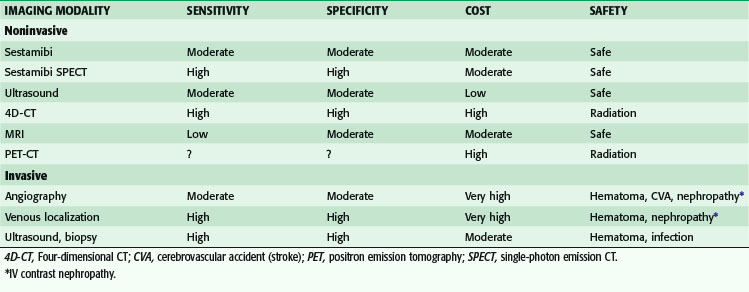
Several noninvasive preoperative localization modalities are available, including technetium-99m (99m Tc) sestamibi scintigraphy, ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), and thallous chloride-201(201Tl) –99mTc-pertechnetate subtraction scanning. Most recently, four-dimensional CT and positron emission tomography (PET)-CT fusion studies have also been used with success for parathyroid localization. There is general consensus that the single best study is sestamibi, especially when combined with SPECT, and it is now the most common nuclear medicine study performed. In 1989, 99mTc, used for cardiac imaging, also was noted to be avidly taken up by parathyroid tissue. The study works by mitochondrial uptake of 99mTc-sestamibi, and parathyroid cells typically have a large number of mitochondria. Sestamibi, a monovalent lipophilic cation, diffuses passively across cell membranes and concentrates in mitochondria. Hence, it is preferentially concentrated in adenomatous and hyperplastic parathyroid tissue because of increased blood supply, higher metabolic activity, and absence of P-glycoprotein on the cell membrane (Fig. 39-4). Sestamibi imaging can be performed preoperatively for MIP planning or on the morning of surgery in the operating room in conjunction with the use of a gamma probe to guide the surgeon during surgery.20
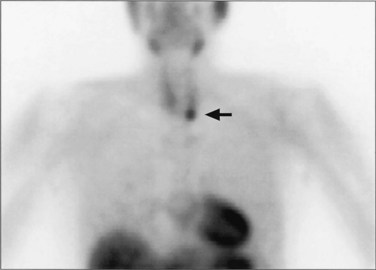
Ultrasound is effective, noninvasive, and inexpensive, but its limitations include operator dependency and restriction to application in the neck because it cannot image mediastinal parathyroid lesions (Fig. 39-5).21 It has a 48% to 74% true-positive rate. Ultrasound often is used in combination with sestamibi, in which case the combined true-positive rate rises to 90%. CT and MRI provide cross-sectional imaging and are useful for visualizing mediastinal tumors and glands within the tracheoesophageal groove. MRI does not involve the use of radiation, and parathyroid adenomas often appear intense on T2-weighted images. CT is less expensive and has a sensitivity of 70% and a specificity of nearly 100%. In a study of 42 surgical patients with primary HPT in which alternative preoperative localization strategies were compared, sensitivity was highest for sestamibi using the 99mTc subtraction technique (95%), followed by 201Tl-99mTc subtraction (86%), CT (83%), and ultrasound (81%).22
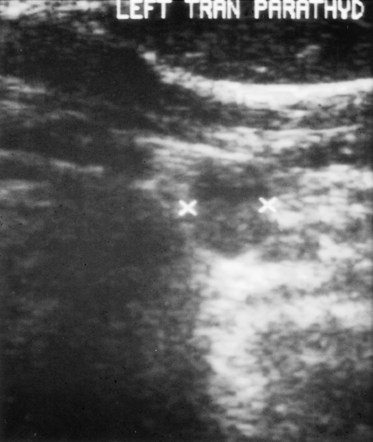
Four-dimensional CT (4D-CT), a novel imaging modality similar to CT angiography, is derived from three-dimensional (3D)-CT scanning with an added dimension from the changes in perfusion of contrast over time. It generates detailed multiplanar images of the neck and allows the visualization of differences in the perfusion characteristics of hyperfunctioning parathyroid glands (i.e., rapid uptake and washout). Therefore, 4D-CT images provide anatomic and functional information (Fig. 39-6). In a study of 75 patients with primary HPT, 4D-CT demonstrated improved sensitivity (88%) over sestamibi (65%) and ultrasonography (57%) when the imaging studies were used to lateralize hyperfunctioning parathyroid glands to one side of the neck.23
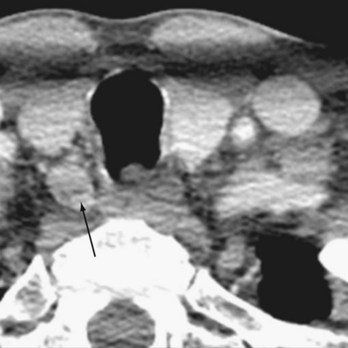
FIGURE 39-6 4D-CT showing increased uptake on delayed phase imaging of a parathyroid adenoma (arrow).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree