The Management of Acute Aortic Dissections
Roy K. Greenberg
Despite medical advances since the inception in 1935 of methods to manage complications from aortic dissections, contemporary treatments are associated with significant morbidity and mortality. Consequently, such therapies are generally reserved for patients experiencing severe symptoms or clinical sequelae from the dissected aorta such as ischemia or rupture. Endovascular aneurysm repair has created a new perspective on the management of patients with aortic disease. There has been significant evolution of the techniques and devices designed to address thoracic aortic pathology; however, endovascular therapy for acute dissections occurred in relatively few institutions and remains unproven. Much activity has occurred following two sentinel papers that appeared in The New England Journal of Medicine in 1999 pertaining to the endovascular management of aortic dissections. Despite improvements in devices and delivery systems, challenges remain, including the ability to deploy prostheses accurately within tortuous arches, iliac access for large sheaths, and the long-term prevention of aortic growth. Controversy has been spawned by the perceived potential to lessen the likelihood of long-term aortic degeneration and thoracoabdominal aneurysm formation if asymptomatic acute dissections are treated in a manner that alters the true and false lumen interface. Indications for therapy classically include acute complications such as ischemia, rupture, and uncontrolled hypertension or rapid aortic growth. Interventions are also performed in the setting of a chronic dissection when significant aortic enlargement has been noted during the follow-up period.
Although overall treatment indications have not changed substantially over the years, the preferred method of therapy has dramatically altered. Unfortunately, the absence of an in vitro or animal model that correlates with human scenarios has made preclinical testing of devices and procedures unhelpful. Consequently, it is generally left to the treating physician to determine which patients will be treated and what type of treatment will be prescribed.
Pathophysiology
The absence of readily definable anatomic or physiologic parameters predictive of acute or long-term complications from aortic dissections has resulted in several studies designed to address this issue. Tear depth, local wall stress, the status of the vasa vasorum, and the angulation of the initial entry tear have been implicated in the propagation and hemodynamic consequences of this disease. In vitro modeling has been helpful to characterize some treatments; however, it is likely that the physical and hemodynamic properties of the aorta in conjunction with more proximal cardiovascular physiologic status will dictate the extent and severity of the dissection.
True lumen compression can induce end organ ischemia by two different methods. The first involves simply decreasing the amount of flow to the distal aorta. This occurs, most commonly, when there is not a large distal fenestration; the mean false lumen pressure is high (in the absence of outflow), resulting in true lumen compression inhibiting distal perfusion (Fig. 13-1). However, if two fenestrations exist, the false lumen may act in the manner of a shunt, preserving adequate distal perfusion. Alternatively, adequate flow may be preserved within the aorta, but ischemia can develop when the dissection plane propagates into one of the branch vessels. An obstruction can develop if the false lumen thromboses or if the dissection flap functions like a valve during the pulsatile aortic flow, occluding the visceral vessel ostium. Both mechanisms can occur simultaneously, and in this circumstance, marked ischemia may develop. Clearly the inherent anatomy and hemodynamic properties of the dissection will help to gauge the optimal form of therapy.
Medical Management
Proper medical management of the patient suffering an acute aortic dissection is paramount to obtaining good interventional results. Varied treatment paradigms exist; however, appropriate diagnostic tests must be obtained in an effort to discriminate dissections proximal and distal to the left subclavian artery, intramural hematomas, and aneurysmal disease. Clearly, the management of acute hypertension, which is nearly always present in dissection patients, is critical. Aggressive beta-blocker therapy dominates contemporary management, and the benefit supersedes treatment with other antihypertensive regimens. However, caution must be exercised when using extreme doses of antihypertensive regimens, as they may be indicative of significant proximal true lumen compression with impending ventricular failure, as we have noted in a number of patients.
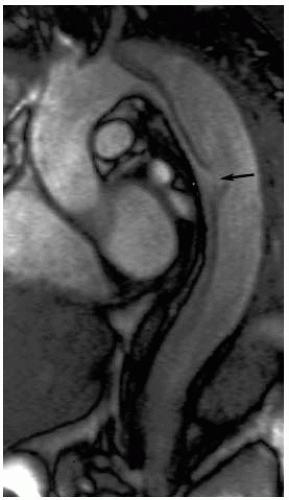
Cross-sectional imaging studies (CT or MRI) and transesophageal echocardiography are extremely accurate in terms of locating the proximal fenestration of aortic dissections. However, the actual imaging algorithms used are critical, and an experienced radiography team is invaluable to patient assessment. Multislice CT scanning has allowed us to obtain neck to pelvis imaging with an accurately timed contrast bolus, and this may be accomplished with a gated technique. MR techniques are also valuable in the evaluation of the physiology of the dissection, as flow can be assessed with the ability to help with the visualize fenestration sites. However, a loss of some axial resolution associated with MR studies may make this technique difficult to use exclusively when planning an endovascular intervention. Regardless of the technique used, attention must be directed at the proximal entry tear of the dissection (Fig. 13-2), true-to-false lumen ratios, detectable fenestrations, branch vessel dissection, the origin of luminal flow for each end organ, and the luminal supply to each of the femoral arteries.
Indications for Therapy: Ischemia, Rupture, and Rapid Growth
Clinically, the decision to intervene on a patient suffering from a type B aortic dissection is based upon the presence of ischemia, rupture, or radiographic evidence of rapid aortic growth. Patients in extremis, such as those with profound ischemia or those that are hemodynamically unstable following an aortic rupture, will clearly fare worse than patients treated prior to the occurrence of such sequelae. However, currently there are no mechanisms capable of predicting which patients will develop these sequelae at the time of initial presentation. The management of patients with visceral and lower-extremity ischemia must address conditions including acidosis, hyperkalemia, and the hemodynamic consequences attributable to lactic acidosis shock or, in the setting of significant true lumen compression, aortic pseudo-occlusion and left heart failure. We have observed the latter situation in patients with deep fenestrations of the proximal aorta following prolonged courses of antihypertensive management. A relatively acute switch from severe hypertension to hypotension can develop necessitating vasopressor support forcing intervention. This may result in a profound reperfusion injury. Thus, at our institution, patients with borderline ischemia are treated aggressively early, particularly if there are clear indications of severe true lumen compression.
Aortic rupture following dissection is more rare than ischemic complications. Radiographically, cross-sectional imaging studies demonstrating pleural fluid must be differentiated from true extravasation of blood. Furthermore, if an endovascular option is to be entertained, imaging studies should be evaluated for the extent of the dissection and potential sites for achieving a seal proximal and distal to the dissection. (Focal dissections of the thoracic aorta resulting in rupture are easily managed with an endovascular approach; however, complex and extensive aortic dissections are better managed with conventional surgery. Permissive hypotension may be advocated until the rupture is excluded. This serves to delay the hemorrhagic process, diminish the need for large amounts of blood products, and provide one with enough time to perform the procedure in the proper setting with an appropriate team.
True/False Lumen Assessment
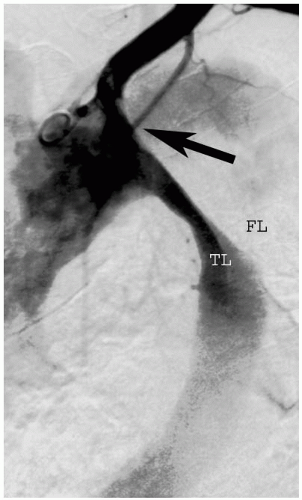
Noninvasive imaging studies will provide the necessary information to direct methods by which procedures or additional studies can be planned. Angiography and intravascular ultrasound have proven to be invaluable tools for determining the extent of dissections, specifically with respect to the luminal relationships to branch vessels. In our experience, the only pre-operative variables that were predictive of the development of an ischemic syndrome were the absolute diameter and relative size of the true lumen (true lumen/false lumen diameter ratio). True-to-false lumen ratios of less than 0.4 were more significantly associated with a visceral ischemic syndrome, especially when associated with a crescent-shaped lumen, while ratios greater than 0.8 were never associated with ischemia. However, these calculations pertain only to acute dissections; chronic dissection ratio calculations were not helpful with respect to outcomes.
Prior to arterial access, the site choice is based upon an assessment of axial images. True lumen access can be obtained from either a brachial or a femoral approach. Most frequently, the femoral artery with a compromised pulse is the most direct route to the true lumen. Alternatively, true lumen access can be obtained from within the
aortic arch using selective catheterization techniques. Regardless of how access is obtained into the respective lumens, interventionalists must be sure of which lumen they are working within and whether the luminal membrane has been traversed with a wire during the intervention.
aortic arch using selective catheterization techniques. Regardless of how access is obtained into the respective lumens, interventionalists must be sure of which lumen they are working within and whether the luminal membrane has been traversed with a wire during the intervention.
It is also critical to detect evidence of dissection propagation into one of the branch vessels. A given branch can render an organ ischemic as a result of false lumen perfusion followed by false lumen thrombosis; this occurs when the dissection flap functions like a valve during the pulsatile aortic flow occluding the visceral vessel ostium, or in the setting of low false lumen flow that mimics a conventional stenotic lesion of the true lumen origin. Although there is overlap of the aforementioned mechanisms, the etiology of the ischemia will dictate the method of intervention.
Failed Therapies
In addition to an assessment of the pathophysiologic mechanisms of aortic dissection, an evaluation of failed therapies is helpful. It is inadvisable to place uncovered stents within the proximal true lumen in an effort to increase true lumen size. The inability of an uncovered stent to direct flow away from the false lumen prevents any passive collapse of the false lumen and relegates any benefit of the aforementioned therapy to shear radial force. Unfortunately, the amount of radial force required to collapse the false lumen may exceed the strength of the aortic wall, thus posing a risk of aortic rupture. Furthermore, it is impossible to apply the force equally in a radial fashion at the level of the primary entry tear, which is often located within the tortuous portion of the aorta. These clinical observations have been supported by animal studies from multiple investigators. Additional concerns regarding the use of balloon or self-expanding stents within either lumen of the dissection pertain to potential difficulties with future interventions that relate to sheath access, device deployment, and prosthesis design.
Endovascular Techniques for Acute Dissections
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree