Open Surgical Treatment of Abdominal Aortic Aneurysms
John A. Curci
Gregorio A. Sicard
Diagnostic Considerations
Because of the generally silent progression of abdominal aortic aneurysmal degeneration, most abdominal aortic aneurysms (AAAs) are currently identified unexpectedly during diagnostic evaluation [such as computed tomography (CT) scanning, magnetic resonance imaging (MRI), or abdominal ultrasound] for other diseases or symptoms. Although focused physical exam can identify up to 90% of aneurysms greater than 5 cm in thin patients, this approach is much less sensitive for a small AAA or any aneurysm in obese patients. Ultrasound screening is the least expensive means by which to diagnose aneurysms of the abdominal aorta, although it is less accurate in the suprarenal aorta and the iliac arteries. Even using ultrasound, it remains controversial whether general population screening is cost effective with the therapies available today. By focusing on high-risk populations, such as elderly male patients with coronary artery disease (CAD) and/or chronic obstructive pulmonary disease (COPD), various studies have shown that selective screening can have a positive impact.
Pathogenesis
Please refer to Chapter 9, “Pathobiology of Abdominal Aortic Aneurysms,” for a detailed discussion on pathogenesis.
Indications and Contraindications
The underlying purpose of aneurysm therapy is to prevent rupture. Currently, the only known means to prevent rupture is to exclude the aneurysmal segment from blood flow. There are two means to exclude the aneurysm: open placement of a synthetic graft or transfemoral (or transiliac) endoluminal stent-graft placement. Each of these techniques has inherent advantages and drawbacks, requiring an individualized approach based on patient anatomy and comorbidities.
Many aneurysms are relatively small with a low risk of rupture. Recent prospective randomized trials in the U.S. (ADAM VA Trial) and the U.K. (UK Small Aneurysm Trial) support nonoperative management for men with aneurysms whose maximum diameter is less than 5.5 cm, provided:
The patient has no symptoms from the aneurysm
There is close follow up of the patient, including semi-annual aneurysm diameter measurements
The growth of the aneurysm remains less than 0.5 cm over any 6-month period.
In both trials, some patients in the surveillance arm underwent elective repair of their AAA before reaching the trial size target of 5.5 cm. Some studies, including the UK Small Aneurysms Trial, have suggested that female gender is an independent risk factor for rupture for aneurysms greater than 5.0 cm in diameter.
Average AAA growth rates are 0.3 to 0.5 cm per year. Unfortunately, discontinuous rather than predictable continuous growth is the norm rather than the exception. Therefore, small aneurysms must be followed for life or until a threshold for operative repair is reached. Although uncommon, known AAAs may become symptomatic prior to actual rupture. Symptoms can include sudden new abdominal or back pain and/or a tender aneurysm. A patient with any of these symptoms should always be considered to be harboring an impending or contained rupture; this makes the rupture a surgical emergency mandating immediate repair.
Anatomic Considerations
Unlike endoluminal aneurysm repair, there are essentially no anatomic constraints to the performance of a successful open aneurysm exclusion. However, a thorough understanding of the individual anatomy of an aneurysm is essential for establishing an appropriate operative approach and plan. The first consideration is the proximal extent of the aneurysm. AAAs that extend to, or are proximal to, the renal arteries may require special modifications of clamp placement and approach and are dealt with in separate chapters.
It is important to also assess the anatomy of the iliac vessels. Concomitant aneurysms of the common iliac artery are quite common and, particularly if greater than 3 cm, should undergo simultaneous exclusion during AAA repair. The hypogastric arteries can also be aneurysmal, although not as frequently as the common iliacs. The approach to these aneurysms can be complex and is addressed in detail elsewhere in the text. The external iliac vessels, on the other hand, are rarely aneurysmal. The common and
external iliac arteries can also be significantly affected by athero-occlusive disease, and bypassing occluded or severely diseased segments is occasionally necessary.
external iliac arteries can also be significantly affected by athero-occlusive disease, and bypassing occluded or severely diseased segments is occasionally necessary.
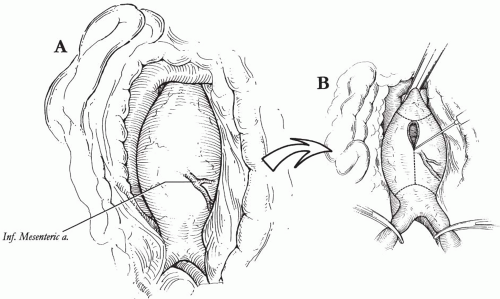
Visceral perfusion may be adversely affected by aneurysm exclusion, and a good understanding of the collateral supply to the viscera is essential. In particular, the inferior mesenteric artery (IMA) and perfusion of the left colon should be carefully assessed, including collateral pathways via the superior mesenteric artery (the Riolan arc and the marginal artery of Drummond) and the hypogastric arteries (hemorrhoidal arteries). In the setting of a normal patent collateral circulation, it is rarely necessary to reimplant the IMA, particularly if it is occluded on pre-operative evaluation. Interruption of collateral pathways by prior surgery or atherosclerosis should be identified, and special consideration should be given to maintaining the remaining supply to the left colon.
Pre-operative imaging can also frequently define certain anatomic variants that can impact the operative plan. In particular, attention should be carefully directed to several renal anomalies, including the location of the renal vein, which may pass posterior to the aorta, the presence of a horseshoe kidney, renal artery occlusive disease, or accessory renal arteries. Prior abdominal or retroperitoneal procedures may impact the relevant anatomy, including renal transplantation, colon resections, and others. The presence of a thickened retroperitoneum on CT scan can signal the presence of an “inflammatory aneurysm,” a variant of the AAA associated with a dense retroperitoneal fibrosis that obliterates normal dissection planes and can make peri-aortic dissection quite treacherous.
All of these anatomic considerations bear on the decision of the appropriate operative approach for an individual patient. The midline transperitoneal approach has historically been the most common. It affords the most flexibility for exposure of the infrarenal aorta, the renal arteries, and both iliac and femoral systems. This approach, however, can be difficult in the setting of prior abdominal operations, juxta- or supra-renal aneurysm extension, horseshoe kidney, peritoneal dialysis, or ascites. Alternatively, the retroperitoneal approach can avoid the intraperitoneal contents entirely and affords improved access to the suprarenal aorta. It also has been documented to reduce gas-trointestinal (GI) and pulmonary complications as well as reduce hospital stay. The limitations of the retroperitoneal approach include poor accessibility to the distal right iliac system and the right renal artery.
Pre-operative Assessment
There are several issues in pre-operative preparation for open AAA repair that must be carefully addressed. In all patients, a thorough history and physical should be performed, including prior abdominal surgery, careful peripheral pulse examination, and auscultation for abdominal and carotid bruits. To complete the general assessment, patients should also be screened with measurement of serum electrolytes, complete blood count, an electrocardiogram (ECG), and a two-view chest x-ray. Approximately 15% of patients with aortic aneurysms also harbor femoral or popliteal aneurysms, many of which can be identified on physical exam. During the exam, suspicion should be high for other manifestations of atherosclerotic disease, including peripheral vascular occlusive disease, coronary disease, and cerebrovascular disease. A carotid duplex study should be performed in patients with a history of prior stroke or transient ischemic attack (TIA) or if a carotid bruit is identified on physical examination. Significant high-grade or symptomatic internal carotid artery stenoses should be considered for endarterectomy prior to elective aneurysm repair.
The strongest risk factors for surgical mortality based on pre-operative comorbidities are listed in Table 17-1. Cardiac complications are the most frequent cause of peri-operative morbidity and mortality. Although some of the risks cannot be modified, advances in pre-operative optimization and post-operative care have reduced the risks of aneurysm repair. A concerted effort of the American College of Cardiology to standardize pre-operative cardiac evaluation for non-cardiac surgery based on best available evidence has resulted in a consensus statement that categorizes the risk of the procedure and the clinical risk. The decision to pursue further cardiac evaluation is based on these categories and the functional capacity of the patient.
Table 17-1 Risk Factors for Operative Mortality Following Elective AAA Repair* | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
The tobacco smoking that predisposes patients to aneurysm formation also predisposes them to significant pulmonary disease. Pre-operative evaluation of patients with a history of COPD should include a room air arterial blood gas measurement and pulmonary function testing, including response to beta-adrenergic agonists. All patients should be encouraged and supported to stop smoking. Patients with severe disease should be optimized with steroid and/or other bronchodilator therapy prior to operation.
Some general pre-operative interventions should be considered in all patients undergoing AAA repair unless directly con-traindicated. These include bowel prep with a mild cathartic to reduce colon caliber and luminal flora, as well as treatment with beta-adrenergic antagonists.
Immediate peri-operative and intra-operative interventions that should be considered include deactivation of any automatic implantable cardiac defibrillation (AICD), placement of a central venous catheter for access and pressure measurements, arterial catheter, urinary catheter, nasogastric tube, upper body warming blanket, and administration of a peri-operative antibiotic. Placement of a warming blanket below the knees can be considered, but great care must be taken to avoid its use during aortic cross-clamping, as local burns may result.
An autotransfusion system can be used in an attempt to limit homologous transfusion. An epidural catheter may be placed to assist in postoperative analgesia, although randomized studies have not been able to clearly support this practice. Pulmonary artery catheters are not beneficial in most patients (and may be harmful) but may have some value in selected very high-risk patients.
Operative Technique
Transperitoneal Approach
The patient is placed on the operative table in supine position with arms extended to the
sides. General anesthesia is induced, and the necessary lines and catheters are placed. The skin is sterilized with a Betadine solution or other antimicrobial solution from a level several centimeters above the xiphoid to the knees. An iodine-impregnated adherent plastic film may be applied to prevent contact of the graft material with the skin surface. The patient is draped widely to allow access to the entire anterior abdominal wall and both femoral arteries. A midline abdominal incision is then made extending from the xiphoid to the pubis. Upon entering the abdominal cavity, the entire contents should be briefly inspected for any pathology not evident on the pre-operative imaging studies. Palpation of the stomach will verify proper placement of the nasogastric tube.
sides. General anesthesia is induced, and the necessary lines and catheters are placed. The skin is sterilized with a Betadine solution or other antimicrobial solution from a level several centimeters above the xiphoid to the knees. An iodine-impregnated adherent plastic film may be applied to prevent contact of the graft material with the skin surface. The patient is draped widely to allow access to the entire anterior abdominal wall and both femoral arteries. A midline abdominal incision is then made extending from the xiphoid to the pubis. Upon entering the abdominal cavity, the entire contents should be briefly inspected for any pathology not evident on the pre-operative imaging studies. Palpation of the stomach will verify proper placement of the nasogastric tube.
The transverse colon is retracted superiorly and the small intestine eviscerated to the right, exposing the ligament of Treitz and the posterior peritoneum overlying the aorta. The proximal jejunum is mobilized by dividing the ligament of Treitz; the posterior peritoneum is then incised to expose the retroperitoneal space to the aortic bifurcation (Fig. 17-1A




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree