Clotting Disorders and Hypercoagulable States
Peter K. Henke
Thomas W. Wakefield
Clotting disorders are extremely common in surgical patients because of disease processes or acquired factors related to patients’ surgery, and/or because these stresses unmask a previously unknown hypercoagulable disorder. Venous thromboembolism (VTE) includes deep vein thrombosis (DVT) and pulmonary embolism (PE), which affect more than 300,000 patients per year, with up to a 15% to 20% mortality rate (primarily PE). DVT is associated with chronic venous insufficiency causing leg swelling, pain, and ulceration affecting up to 30% of patients over an 8-year period of follow up. Thus, it is imperative that measures be taken to reduce this risk of VTE in surgical patients.
Virchow’s triad of stasis, vessel wall injury, and hypercoagulability is still as relevant today as it was in the 1850s. However, the level at which these alterations occur has become better recognized. For example, vessel wall injury is primarily endothelial injury that may promote both the development of thrombosis and the ongoing vessel injury. Underlying hypercoagulable states have been better defined, and risk factors are becoming more apparent as further large population studies and genetic studies are performed. More importantly, the understanding that thrombosis is an inflammatory disorder that promotes both thrombus amplification and vein wall damage is important to keep in mind for future therapies.
With the increased awareness of the fact that many hypercoagulable states are multigenetic, it must also be kept in mind that environmental factors play a key role in when, how, and to what severity these states manifest as a thrombotic clinical event. This is highlighted by the fact that many hemostatic factor-genetic polymorphisms exist, but few have clinically perceptible consequences.
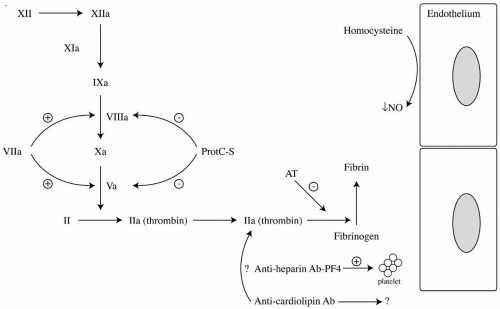
To understand the mechanisms that account for abnormal hypercoagulability, it is important to recall the normal coagulant and fibrinolytic pathways depicted in Figure 4-1. In this schematic, the known potential factor abnormalities in the balance of the system are highlighted. Main anticoagulant mechanisms include antithrombin (AT), which complexes with heparin to inactivate primarily factor IIa, and protein C and protein S, which act as cofactors and together inhibit factor Va and factor VIIIa. This chapter will focus on the evaluation and focused treatment of common disorders as they relate to both pathologic venous and, to a lesser extent, arterial thrombosis. Hematologic fibrinogen/plasmin, and lipoprotein abnormalities will not be discussed.
Acquired Temporal Risk Factors for Venous Thromboembolism
The acquired risk factors for VTE include advanced age, prolonged immobility, obesity, chronic neurologic disease, cardiac disease, pregnancy, oral contraceptives, hormone supplemental therapy, surgery, trauma, and malignancy. Subclinical hypoxemia promotes a procoagulant endothelial response, which may be exacerbated by a postsurgical or elderly state. Specific surgical procedures
that are associated with increased risk include orthopedic procedures, such as knee and hip replacement, and thoracoabdominal operations, as well as urologic and gynecologic procedures. A strong relationship exists between VTE and malignancy, and occult cancer may be present in 0.5% to 5.8% of patients who present primarily with a VTE. An idiopathic VTE is associated with a threefold increased likelihood of presenting with malignancy within 3 years, and 19% of cancer patients have clinical thrombotic events.
that are associated with increased risk include orthopedic procedures, such as knee and hip replacement, and thoracoabdominal operations, as well as urologic and gynecologic procedures. A strong relationship exists between VTE and malignancy, and occult cancer may be present in 0.5% to 5.8% of patients who present primarily with a VTE. An idiopathic VTE is associated with a threefold increased likelihood of presenting with malignancy within 3 years, and 19% of cancer patients have clinical thrombotic events.
Table 4-1 Common Hypercoagulable Syndromes and Diagnostic Tests | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
A careful history and physical allow the physician to best decide who to test for a hypercoagulable state and what tests to order (Table 4-1). Red flags include unusual thrombus location, recurrent idiopathic VTE, VTE in anyone <30 years old, or a woman with multiple stillbirths. Screening of relatives of thrombophilic patients may be worthwhile more so to advise for prophylaxis during high-risk periods, rather than lifelong prophylactic anticoagulant therapy. The presence of these acquired risk factors should alert the physician that greater VTE prophylaxis is needed.
Arterial Thrombosis and Hypercoagulable States
Hypercoagulability and stasis play a lesser role in arterial thrombosis and a major role in VTE. Most of the time, arterial thromboses are associated with atherosclerotic vessel damage, in a setting of specific risk factors, such as diabetes, hyperlipidemia, or tobacco use. Possible genetic risk factors for arterial thromboses include abnormalities of factor VII, fibrinogen, lipoprotein(a), and homocysteine metabolism. While numerous coagulation factor genetic polymorphisms have suggested an increased risk of arterial thrombosis, few have borne out to be predictive in large population analysis. For example, a comparison of specific factor VII polymorphisms and myocardial infarction (MI) has shown protective polymorphisms, but none were associated with increased thrombotic risk. Arterial thrombosis usually manifests with large vessel occlusions that result in MI, stroke, acute and chronic limb ischemia, and other end organ ischemic insults. Primary arterial thrombosis in a healthy vessel is extremely uncommon. Most patients with manifestations of atherosclerosis should be on lifelong antiplatelet therapy.
Loss of Natural Anticoagulant Function
Antithrombin Deficiency
AT is a serine protease inhibitor (also called serpin) of thrombin, kallikrein, and factors Xa, IXa, VIIa, and XIa. It is synthesized in the liver, with a half-life of 2.8 days. AT deficiency, either congenital (autosomal dominant) or acquired, accounts for approximately 1% to 2% of episodes of VTE that may occur at unusual anatomic sites such as mesenteric or cerebral veins. Instances of arterial and graft thrombosis have also been described in AT deficiency. This defect is a significant risk factor for recurrent, life-threatening thrombosis with manifestations early in life, with most cases apparent by 50 years of age. Homozygous individuals with AT deficiency die in utero. Heparin is an anticoagulant because of its ability to potentiate the anticoagulant effects of AT. Causes of acquired AT deficiency include liver disease, malignancy, sepsis, disseminated intravascular coagulation (DIC), malnutrition, and decreased protein production. Nephrotic syndrome may also cause an AT (Mr = 59 kD) deficiency because of the loss of intermediate-sized proteins into the urine along with albumin (Mr = 68 kD).
The diagnosis should be suspected in a patient who cannot be adequately anticoagulated on heparin and/or who develops thrombosis on heparin. The diagnosis is confirmed by measuring AT antigen and activity levels when patients are not taking anticoagulants, including heparin.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree