Chapter 66 The Lymphatics
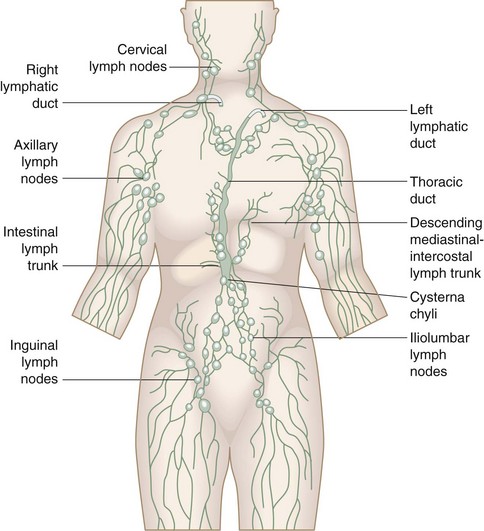
Embryology and Anatomy
From this primordial lymphatic system sprout endothelial buds that grow with the venous system to form the peripheral lymphatic plexus (Fig. 66-1). Failure of one of the initial jugular lymphatic sacs to develop proper connections and drainage with the lymphatic and, subsequently, venous system may produce focal lymph cysts (cavernous lymphangiomas), also known as cystic hygromas.1 Similarly, failure of embryologic remnants of lymphatic tissues to connect to efferent channels lead to the development of cystic lymphatic formations (simple capillary lymphangiomas) that, depending on their location, are classified as truncal, mesenteric, intestinal, and retroperitoneal lymphangiomas. Hypoplasia or failure of development of drainage channels connecting the lymphatic systems of extremities to the main primordial lymphatic system of the torso may result in primary lymphedema of the extremities.
Lymphangiogenesis appears to be regulated by vascular endothelial growth factors C and D (VEGF-C, VEGF-D), their receptor VEGFR-3, and their binding protein, neurophilin-2 (Nrp2). Consistent with these findings, Nrp2-deficient mice have lymphatic hypoplasia and the heterozygous inactivating mutation of VEGFR-3 is found in Chy mice, an animal model of primary lymphedema, which appears to be the underlying problem in patients with Milroy’s disease (congenital familial lymphedema).2
Function and Structure
The lymphatic system is composed of three elements:
Pathophysiology and Staging
Lymphedema is the result of an inability of the existing lymphatic system to accommodate the protein and fluid entering the interstitial compartment at the tissue level.3 In the first stage of lymphedema, impaired lymphatic drainage results in protein-rich fluid accumulation in the interstitial compartment. Clinically, this manifests as soft pitting edema. In the second stage of lymphedema, the clinical condition is further exacerbated by the accumulation of fibroblasts, adipocytes and, perhaps most importantly, macrophages in the affected tissues, which culminate in a local inflammatory response. This results in important structural changes from the deposition of connective tissue and adipose elements at the skin and subcutaneous level. In the second stage of lymphedema, tissue edema is more pronounced, is nonpitting, and has a spongy consistency. In the third and most advanced stage of lymphedema, the affected tissues sustain further injury as a result of the local inflammatory response and recurrent infectious episodes that typically result from minimal subclinical skin breaks in the skin. Such repeated episodes injure the incompetent remaining lymphatic channels, progressively worsening the underlying insufficiency of the lymphatic system. This eventually results in excessive subcutaneous fibrosis and scarring, with associated severe skin changes characteristic of lymphostatic elephantiasis.
Differential Diagnosis
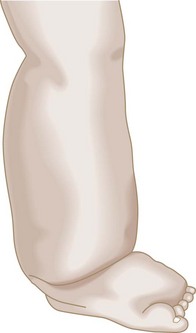
In most patients with second- or third-stage lymphedema, the characteristic findings of the physical examination can usually establish the diagnosis. The edematous limb has a firm and hardened consistency. There is loss of the normal perimalleolar shape, resulting in a tree trunk pattern. The dorsum of the foot is characteristically swollen, resulting in the appearance of the so-called buffalo hump, and the toes become thick and squared (Fig. 66-2). In advanced lymphedema, the skin undergoes characteristic changes such as lichenification, development of peau d’orange, and hyperkeratosis.3 Also, patients give a history of recurrent episodes of cellulitis and lymphangitis after trivial trauma, and frequently present with fungal infections affecting the forefoot and toes. Patients with isolated lymphedema usually do not have the hyperpigmentation or ulceration one typically sees in patients with chronic venous insufficiency. Lymphedema does not respond significantly to overnight elevation, whereas edema secondary to central organ failure or venous insufficiency does.
The evaluation of a swollen extremity should start with a detailed history and physical examination. The usual causes of bilateral extremity edema are of systemic origin; the most common is cardiac failure, followed by renal failure.4 Hypoproteinemia secondary to cirrhosis, nephrotic syndrome, and malnutrition can also produce bilateral lower extremity edema. Another important cause to consider with bilateral leg enlargement is lipedema. Lipedema is not true edema but, rather, excessive subcutaneous fat typically found in obese women. It is bilateral, nonpitting, and greatest at the ankle and legs, with characteristic sparing of the feet. There are no skin changes and the enlargement is not affected by elevation. The history usually indicates that this has been a lifelong problem that runs in the family.
Classification
Lymphedema is generally classified as primary when there is no known cause and as secondary when its cause is a known disease or disorder.5 Primary lymphedema has generally been classified on the basis of the age of onset and presence of familial clustering. Primary lymphedema with onset before the first year of life is called congenital. The familial version of congenital lymphedema is known as Milroy’s disease and is inherited as a dominant trait. Primary lymphedema with onset between the ages of 1 and 35 years is called lymphedema praecox. The familial version of lymphedema praecox is known as Meige’s disease. Finally, primary lymphedema with onset after the age of 35 years is called lymphedema tarda.
Diagnostic Tests
The diagnosis of lymphedema is relatively easy in the patient who presents in the second or third stage of the disease. It can, however, be a difficult diagnosis to make in the first stage, particularly when the edema is mild, pitting, and relieved with simple maneuvers such as elevation.5,6 For patients with suspected secondary forms of lymphedema, computed tomography (CT) and magnetic resonance imaging (MRI) are valuable and essential for the exclusion of underlying oncologic disease states.7 In patients with known lymph node excision and radiation treatment as the underlying problem of their lymphedema, additional diagnostic studies are rarely needed, except as they relate to follow-up of an underlying malignancy. For patients with edema of unknown cause and a suspicion for lymphedema, lymphoscintigraphy is the diagnostic test of choice. When lymphoscintigraphy confirms that lymphatic drainage is delayed, the diagnosis of primary lymphedema should never be made until neoplasia involving the regional and central lymphatic drainage of the limb has been excluded via CT or MRI. If a more detailed diagnostic interpretation of lymphatic channels is needed for operative planning, contrast lymphangiography may be considered.
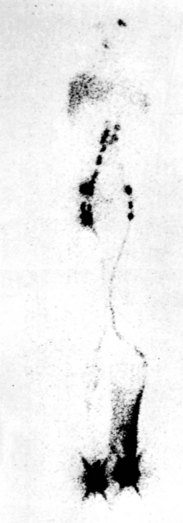
Lymphoscintigraphy (or isotope lymphography) has emerged as the test of choice in patients with suspected lymphedema.7,8 It cannot differentiate between primary and secondary lymphedemas, but it has a sensitivity of 70% to 90% and a specificity of almost 100% in differentiating lymphedema from other causes of limb swelling. The test assesses lymphatic function by quantitating the rate of clearance of a radiolabeled macromolecular tracer (Fig. 66-3). The advantages of the technique are that it is simple, safe, and reproducible, with low exposure to radioactivity (≈5 mCi). It involves the injection of a small amount (2-3 mCi injection in 0.2 mL of saline) of radioiodinated human albumin or 99Tc-labeled sulfide colloid into the first interdigital space of the foot or hand. Migration of the radiotracer within the skin and subcutaneous lymphatics is easily monitored with a whole-body gamma camera, thus producing clear images of the major lymphatic channels in the leg and amount of radioactivity at the inguinal nodes 30 and 60 minutes after injection of the radiolabeled substance in the feet. An uptake value less than 0.3% of the total injected dose at 30 minutes is diagnostic of lymphedema. The normal range of uptake is from 0.6% to 1.6%. In patients with edema secondary to venous disease, isotope clearance is usually abnormally rapid, resulting in more than 2% ilioinguinal uptake. Note that variations in the degree of edema involving the lower extremity do not appear to change the rate of isotope clearance significantly.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree