Minimal sedation (anxiolysis)
Moderate sedation/analgesia (“conscious sedation”)
Deep sedation/analgesia
General anesthesia
Responsiveness
Normal response to verbal stimulation
Purposeful response to verbal or tactile stimulation
Purposeful response following repeated or painful stimulation
Unarousable even with painful stimulus
Airway
Unaffected
No intervention required
Intervention may be required
Intervention often required
Spontaneous ventilation
Unaffected
Adequate
May be inadequate
Frequently inadequate
Cardiovascular function
Unaffected
Usually maintained
Usually maintained
May be impaired
17.2 Anesthesia Induction Sequence
A focused patient history and physical is necessary and required prior to anesthesia, to determine the most appropriate anesthetic management for that individual. Any changes to health condition since previous medical examination or anesthesia should be documented. Past history of any complications or reactions to anesthetics must also be considered. For example, patients with a family history of malignant hyperthermia are at risk of eliciting an episode of malignant hyperthermia when triggering agents such as succinylcholine or volatile anesthetics are administered. All patients undergoing elective surgery should follow the preoperative fasting guidelines established by the American Society of Anesthesiologists (ASAs) [1]. The ASA guideline recommends the minimum fasting duration for solid foods is six hours, breast milk for four hours, and clear fluids such as water for two hours. The NPO guidelines are useful to minimize the risks of gastric regurgitation and thus aspirations of stomach contents into the lungs during the induction of anesthesia. The need for laboratory or diagnostic studies is guided by the preexisting medical condition of each individual patient and type of surgery. Other cardiovascular studies such as electrocardiograms (ECGs), cardiac echocardiography, and/or cardiac stress tests may be considered depending on the given patient’s risk factors.
Common anesthesia techniques include sedation (also known as monitored anesthesia care or MAC) and general anesthesia . Note that the choice of anesthesia technique is again dependent on the patient and/or proposed surgery or procedure. A typical general anesthesia induction sequence for an adult is as follows. After establishing intravenous access and placement of standard ASA [2] monitors, a patient is preoxygenated with 100 % oxygen by a face mask. An induction dose of intravenous medication such as propofol , an opioid such as fentanyl , and a muscle relaxant such as vecuronium are administered to facilitate the subsequent smooth induction of general anesthesia. Once the patient is rendered unconscious and anesthetized, direct laryngoscopy is performed with a laryngoscope and the trachea is intubated with an endotracheal tube. After confirmation of endotracheal intubation by auscultation of lungs and presence of end-tidal carbon dioxide by monitoring, the patient is placed on an anesthesia ventilator and ventilated with a combination of anesthetic gases, oxygen, and/or air. Note that if a total intravenous anesthetic (TIVA) technique with propofol and fentanyl is chosen, anesthetic gases are not administered. A TIVA technique may be chosen for patients who have reactions to volatile anesthetics, such as patients with malignant hyperthermia; the TIVA technique may also be utilized in patients with significant history of postoperative nausea and vomiting following an inhalational anesthetic. The cardiovascular depressant effects of most anesthetics typically become evident during and immediately following induction of anesthesia. Maintaining cardiovascular stability requires (1) careful titration of medications, (2) knowledge of both clinical and basic science in physiology and pharmacology, and (3) diligent monitoring of patients and their vital signs.
For the induction of general anesthesia in young children , a mask induction technique is often utilized. Since placement of an intravenous catheter preinduction may be traumatic to the child and/or difficult due to noncooperation, mask induction with sevoflurane and/or nitrous oxide is frequently employed. Sevoflurane is well tolerated and has a low risk of causing either laryngospasm or bronchospasm and therefore is commonly utilized for mask induction of anesthesia. After placement of ASA monitors, a high concentration of sevoflurane along with oxygen is administered via a face mask until the patient is determined to be anesthetized and unconscious. A peripheral intravenous catheter is then placed to administer additional medication, and a general anesthesia and airway management sequence subsequently follows, similar to the adult patient. Mask induction of anesthesia with sevoflurane is also possible for adults.
It is important to note that direct laryngoscopy and endotracheal intubation can often stimulate the upper and lower airways which, in turn, may cause significant changes in both blood pressure and heart rate, i.e., if airway responses are not blunted. In other words, tachycardia or hypertension can occur during laryngoscopy. Commonly, titration of anesthetics and opioids is administered to blunt these airways and associated sympathetic responses. In some patients (or during specific surgical procedures), it may be required to add invasive monitoring along with the standard ASA monitors. Specifically, such monitors may include (1) invasive arterial lines, (2) central venous catheters, (3) pulmonary artery catheters, and/or (4) transesophageal echocardiography.
17.3 Inhalational Anesthetics
Commonly used inhalational anesthetics include nitrous oxide, isoflurane, desflurane, and sevoflurane (Fig. 17.1). Each of these inhalational anesthetics has an identified specific minimum alveolar concentration (MAC) at which general anesthesia is induced (Table 17.2). MAC is defined as the MAC of an inhaled anesthetic required to prevent movement in 50 % of patients in response to a painful stimulus such as a surgical incision. It is important to note that both infants and children will tend to have a higher MAC requirement than adults, while pregnant women and elderly patients have lower MAC requirements. MAC is additive, that is, 0.5 MAC of nitrous oxide and 0.5 MAC of isoflurane result in 1 MAC total anesthesia. More specifically, the brain anesthetic partial pressure is dependent upon factors such as inspired (F I) and alveolar (F A) concentrations of a given anesthetic gas. Brain (F B) concentration of an anesthetic is dependent upon FA and FI:
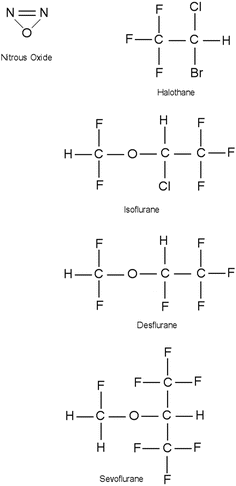
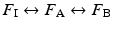

Fig. 17.1
Chemical structure of commonly administered inhalational anesthetics
Table 17.2
Minimal alveolar concentration (MAC) of inhalational anesthetics
Agent | MAC (% of 1 atmosphere) | Vapor pressure (at 20 °C) |
|---|---|---|
Desflurane | 6.0 | 680 |
Halothane | 0.75 | 243 |
Isoflurane | 1.2 | 240 |
Sevoflurane | 2.0 | 160 |
Nitrous oxide | 105 | |
Xenon | 70 |
In general, anesthetic uptake is determined by (1) the relative blood solubility, (2) a patient’s instantaneous cardiac output, and (3) the difference between alveolar and venous partial pressures [3]. Note that the greater the uptake of an anesthetic gas into the blood, the slower the rate of induction. Inhalational anesthetics with lower blood to gas solubility (i.e., desflurane and sevoflurane) will cause a more rapid induction and emergence from general anesthesia, yet higher concentrations of these agents are needed for the MAC requirements.
17.3.1 Blood Pressure and Systemic Vascular Resistance
All volatile anesthetics such as isoflurane, desflurane, sevoflurane, and halothane will cause dose-dependent effects on cardiovascular function. For example, these agents typically cause a dose-dependent decrease in mean arterial blood pressure [4–7]. The relative decrease in mean arterial blood pressure is considered due to decreases in systemic vascular resistance, myocardial contractility, sympathetic output, and/or a combination of the above. In particular, isoflurane, desflurane, and sevoflurane will induce greater decreases in systemic vascular resistance when compared to halothane (Table 17.3). Further, increasing doses of halothane result in small changes in systemic vascular resistance [8] and decreases in mean arterial pressure, yet halothane administration is associated with decreases in cardiac output. In general, volatile anesthetics decrease systemic vascular resistance by causing peripheral vasodilation and thus increasing blood flow to cutaneous and skeletal muscle tissues [4]. It should be noted that nitrous oxide causes minimal alteration of systemic vascular resistance when administered alone. Typically, an initial drop in blood pressure following induction of anesthesia is associated with a decrease in systemic vascular resistance and the associated preload. Lowered blood pressure can be increased by administration of intravenous fluids, placing the patient in a Trendelenburg position, and/or by giving peripheral vasoconstrictors such as phenylephrine or ephedrine.
Table 17.3
Cardiovascular effects of inhalational anesthetics
Heart rate | Blood pressure | Systemic vascular resistance | Cardiac output | Sensitize to epinephrine | Coronary dilation | |
|---|---|---|---|---|---|---|
Desflurane | + | – | – | 0/− | 0/+ | + |
Halothane | 0 | – | 0/− | – | +++ | + |
Isoflurane | + | – | – | – | 0/+ | ++ |
Sevoflurane | 0 | – | – | 0/− | 0/+ | 0 |
Nitrous oxide | + | 0 | 0 | 0 | 0 | 0 |
17.3.2 The Cardiac Conduction System and the Control of Heart Rate
Baroreceptors located near the aortic root, carotid arteries, and other sites detect changes in arterial blood pressure and will automatically affect cardiovascular function. A typical baroreceptor reflex from the carotid artery includes the afferent (cranial nerve IX) and efferent (cranial nerve X) nerves. An increase in arterial blood pressure is detected by the baroreceptor, causing a reflex and nearly instantaneous decrease in the heart rate. A decrease in arterial blood pressure then causes a reflex increase in heart rate, to maintain cardiac output and organ perfusion. Importantly, volatile anesthetics will cause dose-dependent decreases in baroreceptor reflex activity [9]; hence, hemodynamic compensatory responses are attenuated by volatile anesthetics [10, 11]. It is common that alterations in hemodynamics due to volatile anesthetics may require administration of other pressor medications to offset the attenuation of these normal physiologic functions.
Volatile anesthetics may also cause specific cardiac dysrhythmias. Specifically, volatile anesthetics have been reported to slow the rate of sinoatrial node discharge and also increase ventricular and His bundle conduction times [12], which may increase the development of nodal rhythms. Further, volatile anesthetics may increase ventricular automaticity by altering potassium and calcium ion channels [12]. It has been reported that halothane increases the incidence of ventricular dysrhythmia, especially when coadministered with epinephrine; in contrast, the coadministration of epinephrine with isoflurane, desflurane, or sevoflurane has minimal effect on increasing the incidence of ventricular dysrhythmia [13–15]. Furthermore, halothane may blunt the reflex increases in heart rate which typically accompany decreases in blood pressure; it may also slow conduction from the sinoatrial node, resulting in junctional ventricular rhythms. Sevoflurane and desflurane are also known to partially blunt sympathetic baroreflex sensitivity. Importantly, isoflurane is well known to cause significant decreases in systemic vascular resistance and thus blood pressure. Yet, the baroreceptor response remains partially intact, and thus, cardiac output is maintained relatively stable, with isoflurane by associated increases in heart rate.
In patient populations such as young children and the elderly, it is not uncommon to see decreases in heart rate following induction of anesthesia. An anticholinergic agent such as atropine or glycopyrrolate is frequently administered to prevent and/or treat such bradycardia.
17.3.3 Coronary Blood Flow
In general, volatile anesthetics cause a dose-dependent coronary vasodilation, i.e., with isoflurane having a greater effect than halothane [16, 17]. Increasing the concentration of isoflurane increases coronary blood flow, and this also has the potential to cause coronary steal syndrome [18, 19]. Coronary steal is caused by vasodilation of healthy coronary arteries and shunting of blood from myocardium at risk for ischemia to areas not at risk. More specifically, in coronary artery disease, cardiac areas at risk for myocardial ischemia have coronary arteries that are already maximally vasodilated. Desflurane and sevoflurane have not been specifically associated with coronary steal syndrome [20, 21]. Nevertheless, the exact clinical significance of coronary steal in humans remains somewhat unresolved.
17.3.4 Contractility and Cardiac Output
Volatile anesthetics depress myocardial contractility by inducing alterations of calcium ion flux [22]. The mechanism of the negative inotropic effect of volatile anesthetics includes (1) decreased free Ca2+, (2) decreased Ca2+ release from sarcoplasmic reticulum, and/or (3) altered contractile protein response to Ca2+ [22, 23]. Halothane diminishes myocardial contractility more than isoflurane, desflurane, and nitrous oxide. More specifically, isoflurane and sevoflurane cause minimal change in contractility and thus allow for better maintained systemic cardiac output [23]. Due to better cardiovascular stability following either isoflurane or sevoflurane administration compared to halothane, the former agents are typically utilized in patients with congenital heart defects and/or depressed myocardial function.
Due to the simultaneous stimulation of the sympathetic nervous system by volatile agents, the additive myocardial depressant effects of nitrous oxide are usually not evident in healthy individuals. Yet in a compromised and failing myocardium, its depressant effects on contractility become much more evident. More specifically, nitrous oxide has been associated with sympathomimetic effects, as it (1) increases plasma catecholamines, (2) causes mydriasis, and/or (3) induces vasoconstriction of systemic and pulmonary circulations [24]. When nitrous oxide is administered with opioids such as fentanyl, the sympathomimetic effects are minimized or abolished. Therefore, the combined administration of nitrous oxide and opioids may result in a significant decrease in mean arterial pressure and cardiac output.
It is important to note that an abrupt increase in a patient’s desflurane concentrations has been associated with a significant increase in sympathetic output, resulting in increased heart rate and mean arterial pressure. A proposed mechanism for this sympathetic stimulation is that it is due to airway and lung irritations that occur with the administration of high concentrations of desflurane [25]. A smaller increase in sympathetic output is commonly associated with isoflurane administration, whereas sevoflurane (due to lack of airway irritation with its administration) is not associated with any increase in sympathetic output, even with a very rapid increase in concentration. Due to favorable airway properties, sevoflurane is used frequently for inhalational induction of anesthesia in children. High concentrations of sevoflurane (4–8 %) are needed for rapid mask induction and are well tolerated in children. Mask induction of anesthesia can also be used for adults.
17.3.5 Pulmonary Blood Flow
Volatile anesthetics are potent bronchodilators and, in some cases, have been used for the treatment of status asthmaticus [26]. In general, it is considered that volatile anesthetics may cause mild decreases in pulmonary vascular resistance, whereas nitrous oxide administration can cause an increase in pulmonary vascular resistance. Thus, the administration of nitrous oxide in patients with preexisting pulmonary artery hypertension may exacerbate the strain on the right heart by increasing their pulmonary vascular resistance. This elevated pulmonary vascular resistance may also result in right-to-left intracardiac shunting in susceptible patients (i.e., those with specific ventriculoseptal defects). In general, volatile anesthetics also diminish the degree of hypoxic pulmonary vasoconstriction, which may result in hypoxia. It is important that in patients with congenital heart defects (i.e., intracardiac shunts, single ventricle, transposition of great arteries, tetralogy of Fallot; see Chap. 10), the properties of select volatile anesthetics may be critical as they offer better cardiovascular stability.
17.3.6 Cardioprotection/Preconditioning
The potential for myocardial preconditioning with volatile anesthetics has been extensively studied. Importantly, halogenated volatile anesthetics have been shown to provide some degree of cardioprotection against injury associated with ischemia and reperfusion [27–30]. The mechanism of cardioprotection seems to be similar to ischemic preconditioning first described by Murray et al. [31] and thus likely ultimately involves the mitochondrial potassium (KATP) channels [32].
17.3.7 Future Inhalational Anesthetics
Xenon was first used as an anesthetic gas in humans by Cullen and Gross in 1951 [33]. Xenon, an inert gas, has many properties that make it an ideal anesthetic gas; it has very low toxicity and is nonexplosive and nonflammable. The MAC of xenon is approximately 70 %. Its very low blood to gas solubility partition coefficient (0.115) provides fast onset and emergence from anesthesia [34]. Preliminary clinical studies with xenon have shown minimal adverse effects on the cardiovascular system and general hemodynamic parameters [34–36]. More specifically, xenon has been shown to induce minimal effects on alterations in heart rate, coronary blood flow, left ventricular pressure, and atrioventricular conduction time [37]. However, factors that may limit the use of xenon as an anesthetic gas are its cost and requirements for a unique delivery system; xenon must be extracted from the atmosphere, and this process is expensive. Nevertheless, special breathing and delivery systems are in development [38].
It should be specifically noted that all commonly employed volatile anesthetics (i.e., isoflurane, desflurane, sevoflurane, halothane) trigger malignant hyperthermia in susceptible patients. Malignant hyperthermia is an inherited pharmacogenetic disorder that affects skeletal muscle and is characterized by a hypermetabolic response when exposed to a triggering agent such as these volatile anesthetics and/or succinylcholine. Dysregulation of the ryanodine receptor, the calcium release channel of sarcoplasmic reticulum, is typically involved in the induced/unregulated release of calcium from this storage site. Signs and symptoms of malignant hyperthermia include sympathetic hyperactivity, elevated carbon dioxide production, muscle rigidity, hyperthermia, metabolic acidosis, dysrhythmia, and hyperkalemia. Treatment of malignant hyperthermia requires immediate removal of the triggering agent, intravenous administration of dantrolene, and management of the associated symptoms. For more details on malignant hyperthermia, see http://www.mhaus.org/.
17.4 Intravenous Anesthetics
17.4.1 Barbiturates
In general , barbiturates cause central nervous system inhibition (depression) by enhancing the effects of g-aminobutyric acid (GABA) [39]. Barbiturates bind to the GABA receptor complexes which, in turn, increase chloride channel activities and cause subsequent inhibition of the central nervous system. More specifically, the GABA receptor complex has binding affinities for GABA, barbiturates, benzodiazepines, propofol, and/or alcohol [24].
Thiopental (3–5 mg/kg) and methohexital (1.5–2 mg/kg) are common barbiturates used for induction of general anesthesia (Fig. 17.2). After the intravenous injection of thiopental or methohexital, anesthesia is induced rapidly, often within seconds. Yet, the duration of induced anesthesia after a single bolus dose of intravenous barbiturate is short (approximately 5 min), due to rapid redistribution from the brain to other tissues such as muscle and adipose. Importantly, intraarterial injection of thiopental can result in severe vasospasm which may lead to thrombosis, tissue injury, and/or even gangrene. If intraarterial injections do occur, counteractive measures such as sympathetic nerve blocks or administration of papaverine, phenoxybenzamine, or lidocaine may be initiated to decrease induced arterial vasospasm.
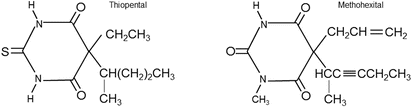

Fig. 17.2
Chemical structure of thiopental and methohexital
The administration of barbiturates is typically associated with decreases in mean arterial pressure which result from both induced vasodilation and decreased myocardial contractility (Table 17.4). Barbiturates have been also shown to cause dose-related myocardial depressions, which are typically not as pronounced as those associated with volatile anesthetics. Yet, barbiturates may cause a small depression of the carotid and aortic baroreceptors and therefore an induced decrease in mean arterial pressure leading to a reflex tachycardia. If intravenous barbiturates are administered slowly, relative hemodynamic stability can be maintained [40], especially in patients with normal intravascular volume status. In contrast, a rapid infusion of barbiturates, especially in hypovolemic patients, may result in significant hypotension. Subsequently, typical increases in heart rate upon barbiturate administration are not present if the baroreceptor reflex is not intact, as in heart transplant patients or in isolated heart preparations. Importantly, barbiturates do not generally sensitize the myocardium to the potential arrhythmic effects of administered catecholamines.




Table 17.4
Cardiovascular effects of intravenous anesthetics
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


