The Diaphragm
Mara B. Antonoff
Jonathan D’Cunha
INTRODUCTION
Separating the thoracic and abdominal cavities, the diaphragm is a dome-shaped muscular structure that serves its key function as the principal muscle of respiration. Through contraction and relaxation, the diaphragm cyclically increases and decreases the volume of the thorax, altering intrathoracic pressure and, ultimately, permitting air entry into the lungs via the establishment of negative pressure. Sustained contraction of the diaphragm serves to increase intraabdominal pressure, useful in generating a Valsalva effect. When functioning well, the diaphragm rarely is the subject of significant clinical attention; however, dysfunction of the diaphragm poses significant and challenging dilemmas to the patient and the surgeon, emphasizing the substantial importance of this musculoaponeurotic structure.
EMBRYOLOGY AND ANATOMY
The embryologic development of the diaphragm occurs between the 7th and 10th weeks of gestation, with the definitive musculotendinous diaphragm incorporating elements of four embryonic precursors: (1) the septum transversum, (2) the pleuroperitoneal membranes, (3) the paraxial mesoderm of the body wall, and (4) the esophageal mesenchyme. From the septum transversum, myoblasts migrate into the right and left pleuroperitoneal membranes, bringing phrenic nerve branches along the way. The majority of the septum transversum gives rise to the nonmuscular diaphragmatic central tendon. The posterolateral portion of the diaphragm is formed by fusion of the dorsal mesentery with the pleuroperitoneal membranes. As these membranous structures are encountered by muscle fibers migrating caudally from the cervical myotomes, the muscular bulk of the diaphragm is formed. Migration of myoblasts into the dorsal mesentery results in formation of the bilateral crura, which originate on the vertebral column and insert into the dorsomedial diaphragm.
While the diaphragm is a fairly consistent organ, with no normal variations in anatomic structure, there are several common abnormalities that result from failed intrauterine development. As the diaphragm originates from several embryonic structures, and normal development requires complex and coordinated execution of a number of vital steps, congenital abnormalities of the diaphragm are well described. Such errors in embryologic development can result in future surgical problems, including congenital diaphragmatic hernias and eventration. Presence or absence of a sac depends on the time point at which the defect occurs, either before or after membranous fusion.
To safely operate on or near the diaphragm, one must have a clear understanding of the anatomic features of the muscular organ, as well as appreciation for the vital structures in close proximity. With its dominantly convex superior surface facing the thoracic cavity and the concave inferior surface facing the abdomen, the diaphragm consists of a mobile component centrally and a fixed component peripherally, which attaches to the inferior margin of the thoracic cage and the superior lumbar vertebrae. The peripheral muscular fibers converge radially at the central tendon. This fibrous, centrally located aponeurosis is composed of the right, left, and middle leaflets, resembling somewhat of a cloverleaf with incomplete divisions. The major muscular elements of the peripheral diaphragm include the sternal, costal, and lumbar groups (Fig. 26.1). The sternal portion is comprised of two muscular attachments to the posterior xiphoid. The costal portion comprises the right and left domes of the diaphragm, attaching to the inferior six ribs and adjacent costal cartilages. The lumbar portion arises from the medial and lateral arcuate ligaments (thickened extensions of the psoas and quadratus laborum fascias, respectively) and attaches to the three most superior lumbar vertebrae.
The left and right crura of the diaphragm arise from the anterior aspects of the lumbar vertebrae. The right crus is longer than the left, and may arise from the first three to four lumbar vertebrae, while the left crus may arise from just the first two to three vertebrae. As the muscle fibers ascend, the medial fibers of both crura decussate just anterior to the aorta. After encircling the esophagus, the muscle fibers insert superiorly into the central tendon of the diaphragm. The central tendon is fused to the inferior aspect of the pericardium via the phrenicopericardial ligaments.
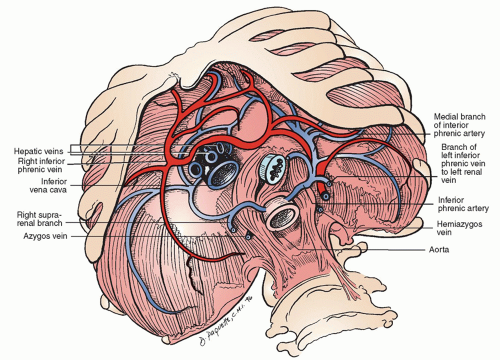
Significant structures pass through three openings: the caval foramen, at T8; the esophageal hiatus, at T10; and the aortic hiatus, at T12 (Fig. 26.2). The caval opening, lying within the central tendon, allows the passage of the inferior vena cava (IVC) as well as terminal branches of the right phrenic nerve and some lymphatic channels. The esophageal hiatus is to the left of midline but is dominantly a formation of the right crus. In addition to the esophagus, this aperture also provides a path for the anterior and posterior vagus nerves, esophageal branches of the left gastric vein and artery, and lymphatics. The aortic hiatus is formed by the crura and the median arcuate ligament, and allows passage of the aorta as well as the thoracic duct.
The major blood supply to the inferior aspect of the diaphragm comes from the left and right phrenic arteries, which come directly from the abdominal aorta in the vicinity of the aortic hiatus (Fig. 26.3). These paired arteries each bifurcate, giving off a large anterior branch that courses along the anterior and superior aspects of the muscle, and merging with the pericardiophrenic artery. The posterior branches of the phrenic artery course laterally and posteriorly and ultimately merge with the intercostal vessels. The pericardiophrenic and intercostal vessels originate from the internal mammary artery. Dominant venous drainage occurs via the
inferior phrenic veins, which drain into the IVC on the right, and both the IVC and the adrenal vein on the left.
inferior phrenic veins, which drain into the IVC on the right, and both the IVC and the adrenal vein on the left.
Motor innervation of the diaphragm comes exclusively from the phrenic nerves (Fig. 26.2). These nerves arise from C3-C5, coming down through the thorax posteriorly, and moving anterolaterally over the pericardium. On the left, the phrenic nerve enters the diaphragm just lateral to the cardiac border and, on the right, the nerve enters the diaphragm just lateral to the caval hiatus. The phrenic nerves, supplying all motor and some sensory innervation of the diaphragm, are made up of four trunks: sternal, crural, anterolateral, and posterolateral. The nerves first give off the sternal branch, then penetrate the diaphragm and course along the inferior surface of the diaphragm. The peripheral portions of the diaphragm receive some sensory fibers from the intercostal and subcostal nerves, as well.
NORMAL FUNCTION
As the chief muscle of inspiration, the diaphragm descends during inspiration and rises during expiration. The right dome may reach as high as the level of the 5th rib and the left dome may ascend to the 5th intercostal space. The exact level of the diaphragm will vary with the phase of respiration, the patient’s position (supine vs. upright), and the volume occupied by the abdominal viscera. Contraction of the diaphragm results in flattening of the domes, increasing the intrathoracic volume, and permitting negative pressure and entry of air into the chest.
For ideal respiratory mechanics, both hemidiaphragms ought to be functionally intact. Unilateral phrenic nerve dysfunction manifests via hemidiaphragm elevation with suboptimal ventilatory movements.
Injury or paralysis of bilateral phrenic nerves can result in significant respiratory compromise, with limited capacity for compensation by the accessory respiratory muscles. In addition to its important role in ventilation, contraction of the diaphragm also has a significant impact on circulatory function, with changes in intraabdominal and intrathoracic pressures aiding in appropriately timed increases in venous return. Further, contraction of the diaphragm may alter intraabdominal pressures such as to facilitate emesis, defecation, and bladder emptying.
SURGICAL CONSIDERATIONS
When operating on or near the diaphragm, familiarity with the path and anatomic location of the phrenic nerves is of great importance. Preservation of diaphragmatic function is best achieved through intraoperative awareness of phrenic innervation and careful placement of incisions when opening the diaphragm for access or excision. Depending on the goals and details of the specific operative procedure, one of several common incisions may prove useful
(Fig. 26.4). Circumferential incisions are placed parallel to the muscle edge, approximately 2 cm from the peripheral margin. Radial incisions may be used, coming from the central tendon outward, but the exact location must be selected such that only the most distal branches of nerve are at risk of transection. A midline incision may be made, either anteriorly or posteriorly, but care must be taken to ligate the left inferior phrenic vein to avoid bleeding from this vessel.
(Fig. 26.4). Circumferential incisions are placed parallel to the muscle edge, approximately 2 cm from the peripheral margin. Radial incisions may be used, coming from the central tendon outward, but the exact location must be selected such that only the most distal branches of nerve are at risk of transection. A midline incision may be made, either anteriorly or posteriorly, but care must be taken to ligate the left inferior phrenic vein to avoid bleeding from this vessel.
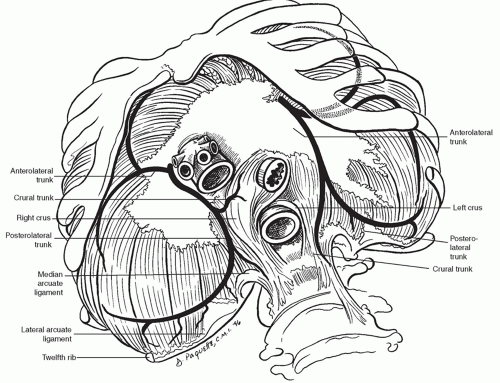 Fig. 26.2. Anatomy of the diaphragm with the branches of the phrenic nerve as viewed from the abdomen. The sternal branch of the phrenic nerve is seen in Figure 26.1. |
IMAGING AND FUNCTIONAL EVALUATION
Prior to operating on the diaphragm, appropriate diagnostic studies should be obtained to confirm presumed diagnoses and to characterize relevant anatomic and physiologic features. Chest radiography serves as a useful initial study for a variety of diaphragmatic abnormalities, and may be followed by additional tests for diagnostic confirmation and further elucidation of specific pathologic features and relevant anatomy. Cross-sectional imaging, via computed tomography (CT) and magnetic resonance imaging (MRI), may provide needed details regarding diaphragmatic defects and neoplastic processes and their relationships with surrounding structures.
When concern has been raised for functional deficits of the diaphragm, the test of choice is termed a “sniff test.” This imaging study, performed under fluoroscopy, involves dynamic imaging of the diaphragm with observed sniffing. In this test, a dysfunctional hemidiaphragm should demonstrate paradoxical movement with the degree of paradoxical movement having clinical relevance. Dynamic breathing MRI can also be used to assess diaphragm function in the setting of concern for diaphragmatic paralysis. Phrenic nerve stimulation may be additionally helpful in evaluating diaphragm function in the setting of neuromuscular disorders.
SPECIFIC DISORDERS AND MANAGEMENT
Congenital Defects
Morgagni Hernia
The hernia of Morgagni is an anterior, retrosternal diaphragmatic defect, occurring between the xiphoid and the costochondral attachments of the diaphragm (Fig. 26.5). Relatively rare, this embryologic failure of myoblast migration accounts for less than 1 in 50 cases of diaphragmatic defects. With this defect, abdominal contents tend to herniate on the patient’s right, with the left-most aspect of the defect occupied by pericardium. While this congenital defect is present at birth, symptoms are relatively minor, and, oftentimes,
patients do not present until adulthood. Increased abdominal pressure, such as with obesity or pregnancy, tends to precipitate increased symptoms, frequently leading to the diagnosis. These symptoms may range from vague fullness to bowel obstruction, and in some patients the defect may be identified incidentally upon radiographic evaluation performed for unrelated indications. Regardless of the extent of symptoms, operative repair is performed for all appropriate surgical candidates in order to prevent complications of obstruction and ischemia.
patients do not present until adulthood. Increased abdominal pressure, such as with obesity or pregnancy, tends to precipitate increased symptoms, frequently leading to the diagnosis. These symptoms may range from vague fullness to bowel obstruction, and in some patients the defect may be identified incidentally upon radiographic evaluation performed for unrelated indications. Regardless of the extent of symptoms, operative repair is performed for all appropriate surgical candidates in order to prevent complications of obstruction and ischemia.
Repair of Morgagni hernias is typically performed via an abdominal approach. Either a midline or subcostal incision will facilitate appropriate visualization and exposure. Laparoscopic repair is also well described. For those circumstances in which herniated structures appear to be fixed high in the chest, a transthoracic approach may be required for adhesiolysis and sac reduction. After gaining access, attention should be directed toward reducing contents of the sac using gentle traction, taking care to avoid injury to involved organs. The sac is then resected. Following reduction and resection of the hernia sac, repair of the diaphragm is performed. The defect may, potentially, be closed primarily if there remains a reasonable margin of muscular tissue around the defect. Closure is performed with heavy nonabsorbable suture placed in an interrupted fashion (Fig. 26.6). In the absence of a complete muscular rim, the defect will require attachment of the free muscle edge to the costal margin. One should also note that if a primary repair results in excessive tension, nonabsorbable mesh or a polytetrafluoroethylene (PTFE) patch should be placed to close the defect between the diaphragmatic rim and the chest wall. If the pleural space is entered, a chest tube should be placed at the end of the procedure.
Bochdalek Hernia
Congenital failure of the closure of the pleuroperitoneal canal results in a posterolateral diaphragmatic defect, permitting herniation of the foregut structures as they return into the abdominal cavity (Fig. 26.5). The majority of these defects tend to be left sided (>85%), and they are often associated with significant cardiac anomalies. Major morbidity results from the space occupation in the chest by the abdominal organs, ultimately hindering normal lung development. The subsequent pulmonary hypoplasia renders many of these patients critically ill, during the delivery and neonatal periods, ultimately causing substantial morbidity and mortality.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree