The Burden of Increasing Worldwide Cardiovascular Disease: Introduction
It is widely acknowledged that heart disease and stroke are the leading causes of death and disability in high per capita income countries (HIC).1 What is less appreciated is that this holds true for the low- and middle-income countries (LMIC) as well.1 We are in the midst of a true global cardiovascular disease (CVD) epidemic.2 CVD is responsible for about 30% of all deaths worldwide each year.3 Of note, nearly 80% of these deaths occur in LMIC, and half occur in women. Indeed, CVD is the leading cause of mortality in every region of the world with the sole exception of sub-Saharan Africa, where infectious diseases are still the leading cause. It is anticipated that even in sub-Saharan Africa, CVD will be the leading cause of mortality within the next few years.
This chapter describes the current global burden of CVD and its risk factors, emphasizing the evolution of the CVD epidemic in developing countries and its contributory factors. Furthermore, the projected trends in the global burden of CVD over the next 2 decades are elucidated, and ongoing efforts by the world community (including the World Health Organization [WHO]) to combat and contain the current epidemic are outlined. The broad term CVD includes coronary heart disease (CHD, which includes myocardial infarction [MI], angina, coronary insufficiency, and coronary death), cerebrovascular disease (including stroke and transient ischemic attacks), peripheral vascular disease, congestive heart failure (CHF), hypertension, and valvular and congenital heart disease.
The World in Transition: Implications for Cardiovascular Disease
The past 2 centuries have witnessed major changes in the demographic characteristics of the human population.4 This transformation (termed demographic transition) involved a progressive change from very high birth and infant mortality rates to low ones. This change was accompanied by a shift from low population growth rates through an intermediate phase of high growth rates, with a consequent major increase in total population. This was then followed by a reversal to low or zero growth rates. The demographic transition results in a conversion of the age distribution of the population from one with a preponderance of young to one with nearly equal representation of all age groups.
The demographic transition has been driven by the most dramatic improvements ever in the history of human health. Improvements in sanitation, nutrition, and infectious disease control and advances in perinatal care have resulted in lower infant and child mortality rates and an enhancement of overall life expectancy. The improvement in life expectancy began in Europe in the late 19th century and by the second half of the 20th century had spread to the rest of the world. Life expectancy at birth has increased from a global average of 46 years in 1950 to 67 years in 2009.5
LMIC have been undergoing rapid industrialization, urbanization, economic development, and market globalization over the past 4 decades.1 As a consequence, standards of living have increased but so have inappropriate dietary patterns and physical inactivity. The nutritional status of populations has been adversely influenced by the aforementioned changes, a phenomenon referred to as nutritional transition.6
Globalization has resulted in the expansion of food economies from local to broad-based ones in which there is easy access to large amounts of unhealthy food products. The shift in dietary patterns comprises a change in all three major food constituents (namely, fats, proteins, and carbohydrates).7 Traditional local diets rich in fiber and low in fat content are being replaced by cheap, energy-dense, saturated fat-laden, micronutrient-poor foods. Vegetarian diets characterized by high intake of plant proteins have been substituted with nonvegetarian diets rich in animal proteins. Complex carbohydrates in diets have been supplanted by refined carbohydrates with a high glycemic index.7 The overall increased caloric consumption occurs in a milieu of reduced energy expenditure caused by sedentary lifestyles with the advent of motorized transport and increased use of labor-saving home and office appliances. Additionally, leisure time physical activities have given way to physically undemanding pastimes, including watching television.
These changes in dietary and lifestyle patterns foreshadow in LMIC an increasing burden of diet-related diseases, including obesity, dyslipidemia, diabetes mellitus, hypertension, and eventually CVD, along with various forms of cancer. The landmark 52-nation INTERHEART study examined local dietary patterns and found a graded relationship between acute MI and “Western” diets characterized by increasing quantities of fried foods, salty snacks, eggs, and meat. “Prudent” diets high in fruit and vegetables were found to reduce the risks of acute MI.8 In essence, although referred to under the umbrella of noncommunicable disease, CVD is to some extent a communicated disease, spread by the forces of globalization.
The previously mentioned demographic, economic, and nutritional changes inexorably lead to major changes in the patterns of human diseases, a phenomenon referred to as epidemiologic transition.4 Epidemiologic transition is characterized by a progressive shift from a predominance of nutritional deficiencies and infectious diseases to those categorized as degenerative (ie chronic diseases such as CVD, cancer, and diabetes).4
Challenges of the Cardiovascular Disease Epidemic in Developing Countries: Differences from Developed Countries
Although the determinants of the health transition in developing countries are similar to those in the developed countries, it is important to emphasize that the dynamics of health transition are different.1,9,10
Temporal compression: Although in HIC, the epidemic of CVD took decades to establish itself, in LMIC, the CVD epidemic is occurring over a compressed time frame, partly related to the rapidity of globalization. Compression of the time course of the epidemic requires a greater intensity of public health response.
Macroeconomic forces: The CVD epidemic in LMIC occurs in settings of poverty and international debt, which may restrict fiscal policy latitude with respect to public health. An accentuating factor is the easy access to low-cost cigarettes in transitioning LMIC. Tobacco is often a cash crop with intensive labor requirements, which makes regulatory control of the lucrative tobacco industry unpopular among domestic constituencies.
Microeconomic forces: Individual responses to comprehend and combat the CVD epidemic in LMIC are restricted by limited education, limited health education, limited financial resources, and limited access to health information and treatment. An additional aggravating factor is that CVD affects individuals at an earlier age in developing countries, resulting in loss of economic productivity, “trapping” individuals and their dependents in a cycle of poverty.
Double epidemiologic burden: LMIC are facing a dual burden of communicable and noncommunicable diseases, resulting in difficult allocation decisions under severe fiscal constraint.
Data gap: The global response to the ongoing epidemic is challenged by a lack of the necessary infrastructure to define, characterize, and track the CVD epidemic in LMIC.
Social knowledge gap: The societal response to the CVD epidemic lags because of the insidious, often invisible toll of CVD risk factors, a lack of popular awareness, and the prevalent belief that CVD is disease of HIC.
Novel epidemiologic profile: It is important to note that for several countries in Asia and Africa, increases in blood pressure and tobacco use preceded the impact of nutrition transition by decades. This has resulted in a differing CVD profile with higher levels of stroke but relatively low levels of CHD. These variant CVD patterns underscore an opportunity to implement strong CHD preventive programs focusing on nutrition and physical activity and aggressive control of blood pressure and tobacco use.
Measuring the Burden of Disease: The Global Burden of Disease Project and the Concept of Disability-Adjusted Life-Year
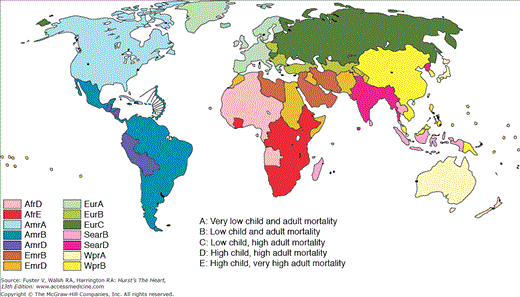
Because of a lack of reliable evidence to inform global health policy, the World Bank commissioned the first Global Burden of Disease Study (GBD, 1990), which was executed by the Harvard School of Public Health in collaboration with the WHO.11 The continuing efforts of the GBD project have generated the most comprehensive set of estimates of morbidity and mortality caused by various disease conditions according to age, gender, and region. GBD publications divide the world into WHO regions with subdivisions for HIC and LMIC (Fig. 2–1).11
Figure 2–1
World Health Organization (WHO) subregions for global burden of disease. For geographic disaggregation of the global burden of disease, the six WHO regions of the world have been further divided into 14 subregions based on levels of child (younger than age 5 years) and adult (15-59 years) mortality for WHO member states. The classification of WHO member states into the mortality strata were carried out using population estimates for 1999 (United Nations population division, 1998) and estimates of 5q0 and 45q15 based on WHO analyses of mortality rates for 1999. Five mortality strata were defined in terms of quintiles of the distribution of 5q0 and 45q15 (both genders combined). Adult mortality 45q15 was regressed on 5q0, and the regression line was used to divide countries with high child mortality into high adult mortality (stratum D) and very high adult mortality (stratum E). Stratum E includes the countries in sub-Saharan Africa, where HIV/AIDS has had a very substantial impact. Adapted from Mathers CD, Stein C, Fat DM, et al. Global Burden of Disease 2000: Version 2 Methods and Results. Global Program on Evidence for Health Policy Discussion Paper No. 50. Geneva: WHO, October 2002.
The GBD project introduced the disability-adjusted life-year (DALY) to quantify the burden of disease.13 The DALY is a health gap measure that summates the potential years of life lost because of premature death and the years of “healthy” life lost in states of less than full health, broadly termed disability. A “premature” death is defined as a death that occurs before the age to which the person could have been expected to survive if he or she was a member of a standardized model population with a life expectancy at birth equal to that of the world’s longest-surviving population, Japan. Thus, one DALY can be thought of as 1 lost year of healthy life. The burden of disease is the gap between current health status of a population and an ideal situation in which everyone lives into old age free of disease and disability.
The WHO has undertaken an assessment of the GBD for the year 2005 (GBD, 2005) with the specific objectives to quantify the burden of premature mortality and disability by age, gender, and WHO region for major causes or groups of causes to analyze the contribution of selected risk factors to this burden to develop various projection scenarios of the burden of disease and to develop tools to enable broader research into burdens of disease.14,15 Detailed tables for health related outcomes are available on the WHO Web site at .
Cardiovascular Disease
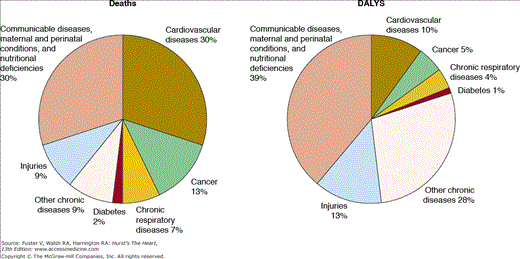
CVD is the leading cause of mortality worldwide, responsible for one-third of all deaths (Fig. 2–2).16 According to WHO estimates, 17 million people died of CVD in 2004.17 Developing countries contributed 80% of CVD deaths. There is considerable variation in CVD incidence, prevalence, and mortality rates across WHO regions (Tables 2–1 and 2–2) and across countries (Fig. 2–3).18 In terms of combined morbidity and mortality, CVD accounted for 151 million DALYs lost worldwide in 2004.17 Potential reasons for such variation include differing stages of epidemiologic transition in various countries, varying environmental effects caused by dissimilar burdens of CVD risk factors, inherent genetic differences, and distinct early childhood programming influences.9,19
| CVDa | AFR | AMR | EUR | SEAR | WPR | EMR | World |
|---|---|---|---|---|---|---|---|
| Mortality (thousands) | |||||||
| CHD | 346 | 925 | 2296 | 2011 | 1029 | 579 | 7198 |
| Cerebrovascular | 425 | 461 | 1364 | 1074 | 2128 | 254 | 5712 |
| HTN heart disease | 78 | 151 | 179 | 156 | 316 | 103 | 987 |
| Rheumatic | 11 | 10 | 30 | 129 | 93 | 25 | 298 |
| Inflammatory | 53 | 69 | 123 | 74 | 87 | 33 | 440 |
| Other CVD | 262 | 353 | 775 | 431 | 441 | 169 | 2438 |
| All CVDs | 1175 | 1969 | 4767 | 3875 | 4094 | 1163 | 17073 |
| Total Burden, DALYs (millions) | |||||||
| CHD | 3.51 | 6.52 | 16.83 | 21.58 | 7.88 | 6.15 | 62.59 |
| Cerebrovascular | 4.88 | 3.99 | 9.53 | 9.6 | 15.84 | 2.7 | 46.59 |
| HTN heart disease | 0.82 | 1.1 | 1.14 | 1.69 | 2.3 | 0.94 | 8.02 |
| Rheumatica | 0.32 | 0.14 | 0.41 | 2.49 | 1.23 | 0.59 | 5.19 |
| Inflammatory | 1.15 | 0.84 | 1.45 | 1.47 | 0.78 | 0.53 | 6.24 |
| Other CVD | 3.56 | 2.63 | 5.4 | 5.23 | 3.73 | 2.19 | 22.75 |
| All CVDs | 14.24 | 15.22 | 34.76 | 42.06 | 31.76 | 13.1 | 151.38 |
| CVD | AFR | AMR | EUR | SEAR | WPR | EMR | World |
|---|---|---|---|---|---|---|---|
| Annual Incidence in 2004, thousands | |||||||
| Cerebrovasculara,b | 706 | 855 | 1987 | 1772 | 3251 | 435 | 9017 |
| Point Prevalence in 2004, thousands | |||||||
| Cerebrovasculara,c | 1549 | 4770 | 9566 | 4409 | 9059 | 1068 | 30,471 |
| CHFc | 1454 | 2881 | 4640 | 4657 | 4065 | 1265 | 18,999 |
| Anginac | 1989 | 6320 | 17,220 | 15,999 | 8229 | 4075 | 53,951 |
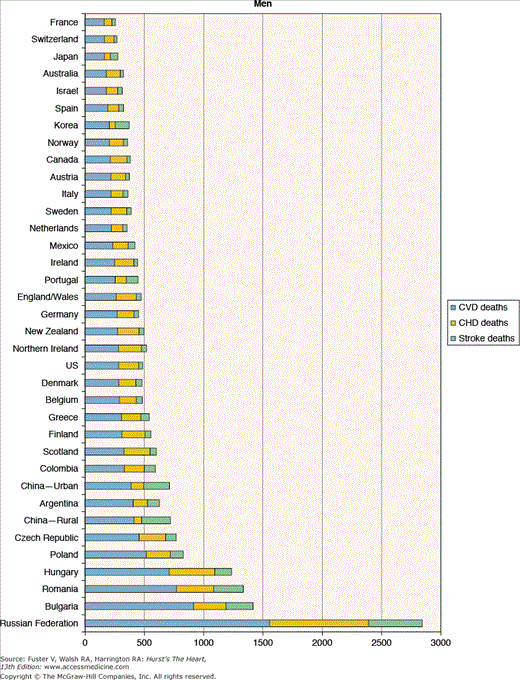
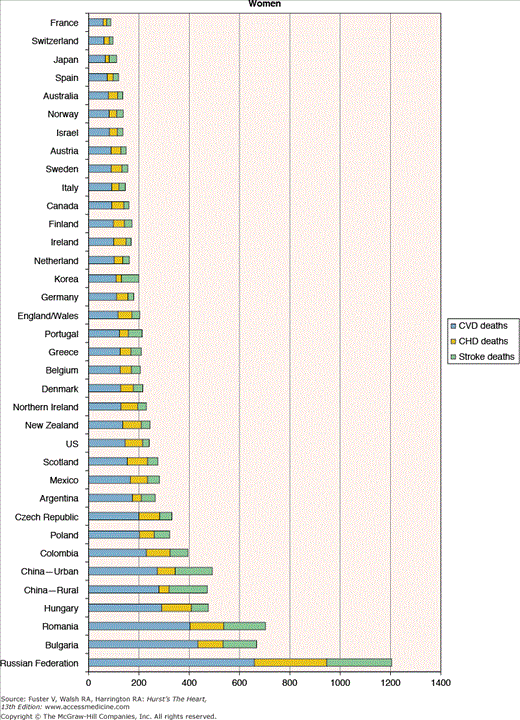
Figure 2–3
Heterogeneity in cardiovascular disease mortality rates (per 100,000 population) across countries. CHD, coronary heart disease; CHF, congestive heart failure. Reproduced with permission from American Heart Association. International Cardiovascular Disease Statistics and Burden of CVD Risk Factors in the US. Available at http://www.americanheart.org/downloadable/heart/1140811583642InternationalCVD.pdf. © 2006, Copyright American Heart Association.
The WHO projections indicate that a pattern of premature CVD mortality is likely to persist and may accentuate further in developing countries (Table 2–3). In 2006, CVD was more prevalent in China and India than in all developed countries combined.17 By 2020, the WHO estimates that there will be nearly 20 million CVD deaths worldwide every year, and the number will increase to 24 million by 2030.20 Developing countries will account for 70% of deaths caused by coronary heart disease and 75% of deaths caused by stroke.17
| Global Burden | 2010 | 2020 | 2030 |
|---|---|---|---|
| CVD Mortality | |||
| Annual, millions | 18.1 | 20.5 | 24.2 |
| % of all deaths | 30.8 | 31.5 | 32.5 |
| CHD death, % of all | |||
| Men | 13.1 | 14.3 | 14.9 |
| Women | 13.6 | 13.0 | 13.1 |
| Stroke death, % of all | |||
| Men | 9.21 | 9.8 | 10.4 |
| Women | 11.5 | 11.5 | 11.8 |
| CVD DALYs | |||
| Annual, million | 153 | 169 | 187 |
| % of all DALYs | 10.4 | 11.0 | 11.6 |
| Global rank | 3rd: CHD 5th: Stroke | 3rd: CHD 4th: Stroke | 3rd: CHD 4th: Stroke |
| Rank in developing countries | 4th: CHD 8th: Stroke | 3rd: CHD 6th: Stroke | 3rd: CHD 5th: Stroke |
Since 1900, CVD has been the leading cause of death in the United States every year (except for 1918).21 In 2005, CVD accounted for 34.2% of all deaths. In fact, CVD claims more lives each year than the next four leading causes of death combined. According to American Heart Association (AHA) estimates, approximately 2400 Americans die of CVD each day, an average of one death every 37 seconds.21 The overall death rate per 100,000 from CVD in the United States was 278.9 in the year 2005. CVD death rates are higher for men than they are for women, and for blacks compared to whites; in 2005, CVD death rates were 324.7 for white men versus 438.4 for black men. Among women, rates ranged from 230.4 for white women to 319.7 for black women. In the United States, there are marked regional disparities, with the southeast experiencing the highest CVD mortality rates. The pathogenesis of ethnic and regional disparities in CVD morbidity and mortality are multifactorial and are discussed later in this chapter.
It is estimated that 80 million Americans have one or more type of CVD.21 Prevalence of CVD varies from about 46% in black women and men to 38% in white men and 33% in white women to 33% of Mexican American women and 26% of Mexican American men down to 11% of Native Americans, 9% of non-Mexican Hispanics, and 6.5% of Asians.21 CVD is an expensive illness. The estimated direct and indirect costs associated with CVD were about $403 billion for the year 2006.
Data from the Framingham Heart Study, a predominantly white cohort followed from 1948 (original cohort) and 1971 (offspring cohort), provide estimates of CVD event rates. The average annual rates of first major CVD events increase with age, rising from seven per 1000 men at ages 35 to 44 years to 68 per 1000 at ages 85 to 94 years (Table 2–4). For women, CVD rates comparable to men are achieved 10 years later in life, with the gender difference in rates narrowing with advancing age. CHD is the predominant cardiovascular event, comprising more than half of all CVD events in men and in women under age 75 years (Table 2–5). The proportions of cardiovascular events caused by CHD decline with age because of the increasing proportions of stroke and CHF.
| Cardiovascular Disease (all types) | Coronary Heart Disease | Stroke and Transient Ischemic Attack | Congestive Heart Failure | |||||
|---|---|---|---|---|---|---|---|---|
| Age (y) | Men | Women | Men | Women | Men | Women | Men | Women |
| 35-44 | 7 | 3 | 4 | 1 | b | b | b | b |
| 45-54 | 15 | 7 | 10 | 4 | 2 | 1 | 2 | 1 |
| 55-64 | 26 | 15 | 21 | 10 | 4 | 3 | 4 | 2 |
| 65-74 | 39 | 24 | 24 | 14 | 11 | 8 | 9 | 6 |
| 75-84 | 59 | 40 | 33 | 18 | 20 | 15 | 18 | 12 |
| 85-94 | 68 | 63 | 35 | 28 | 12 | 25 | 39 | 31 |
| 35-64c | 17 | 9 | 12 | 5 | 2 | 2 | 2 | 1 |
| 65-94c | 44 | 30 | 27 | 16 | 13 | 11 | 12 | 9 |
| Cardiovascular Disease (N) | Coronary Heart Disease (%) | Stroke and Transient Ischemic Attack (%) | Congestive Heart Failure (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Age (y) | Men | Women | Men | Women | Men | Women | Men | Women |
| 35-54 | 352 | 200 | 76.1 | 60.9 | 9.6 | 13.8 | 5.0 | 10.6 |
| 55-64 | 437 | 329 | 69.9 | 62.2 | 11.1 | 14.6 | 5.2 | 8.7 |
| 65-74 | 358 | 364 | 57.9 | 53.6 | 20.8 | 24.5 | 7.2 | 8.4 |
| 75-94 | 199 | 312 | 51.0 | 39.3 | 26.0 | 35.0 | 13.5 | 16.8 |
The long-term risk of developing CVD in an individual is best described by the lifetime risk statistic (ie, the probability that an individual will develop CVD over the course of his or her lifetime). Lifetime risk estimates are computed as cumulative incidence of the disease, usually for conveying to the general public the risk of experiencing a disease event from age 40 to 90 years. The lifetime risk of developing CVD at age 50 years is estimated to be one in two for men and two in five for women.22
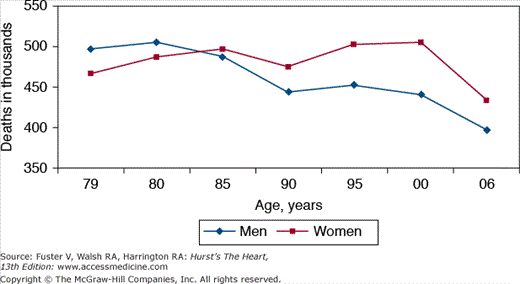
CVD mortality has declined in the United States progressively since about 1940, with sustained long-term declines since the mid-1960s (Fig. 2–4).23,24 CVD mortality decreased by just less than 1% per year in the 1950s and 1960s. The decline became steeper in the 1970s, with the rate falling 3% per year since then. From 1995 to 2005, CVD death rates fell 26.4%, and actual CVD deaths declined by 9.6%.21 Of note, although the initial rapid decrements in CVD mortality rates were consistent across racial groups, since the mid-1980s, a divergence in CVD trends has been noted, with black men experiencing a slower decline than white men.24
Coronary Heart Disease
Numerous epidemiologic investigations have characterized the risk factors for CHD. Age, male gender, elevated low-density lipoprotein (LDL-C) cholesterol levels, low high-density lipoprotein (HDL-C) cholesterol levels, diabetes mellitus, and smoking are key risk factors for CHD.25-32 Risk scores have been developed that can aid the determination of CHD risk.31,33 The Framingham risk score31 is one of the most popular ones but requires recalibration when used to estimate absolute CHD risk in other populations. There is increasing awareness that obesity is a key risk factor that antedates and promotes several CHD risk factors. Obesity does not appear in many risk prediction tools because the risks are partly mediated through other risk factors. Recent data from the Framingham Heart Study indicate that 90% of CHD events occur in individuals with elevated levels of on established risk factor.34 Results from INTERHEART demonstrate that nine risk factors (high-risk diet, lack of alcohol intake, physical inactivity, abdominal obesity, diabetes, hypertension, smoking history, current smoking, and ApoB/ApoA1 ratio) accounted for more than 90% of population attributable risk. It is heartening to note that many, if not all, of these risk factors are modifiable. Men experienced CHD 9 years earlier than women, largely because of risk factors burden.35
In 2004, CHD caused 7.2 million deaths worldwide and accounted for a loss of 63 million DALYs (see Table 2–1). Each year, there are about 5.8 million new CHD cases, and about 40 million individuals with prevalent CHD are alive today.
Data collected from 35 populations that were part of the Monitoring Trends and Determinants in Cardiovascular Disease (MONICA) Project during the mid-1980s until the mid-1990s reveal substantial heterogeneity in coronary event rates (MI and coronary deaths) across countries.36 Thus, the coronary event rate (per 100,000) in men varied 10-fold, being highest in Finland (835 in North Karelia) and lowest in China (81 in Beijing). Likewise, an eight-fold variation in coronary event rates was observed among women; the highest event rate was in the United Kingdom (265 in Glasgow), and the lowest rates (35) were noted both in Spain and China.
The MONICA Project tracked coronary event rates, risk factors, and coronary care in predefined populations in 31 countries over a 10-year period from the mid-1980s to the mid-1990s.37 On average, whereas coronary event rates decreased from 23 (women) to 25 (men)%, CHD mortality rates reduced by 34 (women) to 42 (men)% during the observation period.38,39 The greatest decline in coronary event rates in men occurred in north European populations—namely, Finland, which had the highest levels at the beginning of the observation period, and Northern Sweden. Populations experiencing notable increases in coronary event rates were predominantly from central and Eastern Europe and Asia, although the general pattern of increases and decreases appeared to be less consistent in women.
In regions where coronary mortality rates were decreasing, it is estimated that improvements in survival contributed one-third and changes in heart attack rates accounted for two-thirds, on average, of the total change in survival rates.38,39 These data underscore the importance of both the prevention of heart disease and improved care of acute events in determining CHD mortality rates at the population level.
The decline in CHD mortality in HIC is in sharp contrast to future projections for the LMIC. Between 1990 and 2020, CHD mortality is expected to increase by 120% in women and by 137% in men in LMIC. It is estimated that the annual number of deaths caused by CHD in LMIC will increase to 11.1 million in 2020. CHD mortality will triple in Latin America, the Middle East, and sub-Saharan Africa over the next 2 decades. By contrast, in HIC, CHD mortality is projected to increase by about 30 % to 60%, driven by aging of the population.40
In the United States, an estimated 16.8 million people have CHD, about half of whom have acute MI and half have angina pectoris (Table 2–6).21 Roughly 8.7 million men and 8.1 million women have CHD. Prevalence by state ranges within the United States from 2.3% to 6.0% for MI and from 2.4% to 7.6% for angina or CHD. The estimated direct and indirect costs associated with CHD were about $165.4 billion for 2006.21
| Coronary Heart Disease | Myocardial Infarction | Stroke | Congestive Heart Failure | Cardiovascular Diseaseb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence | Incidence | Mortality | Prevalence | Incidence | Mortality | Prevalence | Incidence | Mortality | Prevalence | Mortality | Prevalence | Mortality | |
| Total | 16,800,000 | 1,260,000 | 445,700 | 7,900,000 | 935,000 | 151,000 | 6,500,000 | 795,000 | 143,600 | 5,700,000 | 292,200 | 80,000,000 | 864,500 |
| Men | 8,700,000 | 740,000 | 232,100 | 4,700,000 | 565,000 | 80,100 | 2,600,000 | 370,000 | 56,600 | 3,200,000 | 126,200 | 38,700,000 | 409,900 |
| Women | 8,100,000 | 515,000 | 213,600 | 3,200,000 | 370,000 | 70,900 | 3,900,000 | 425,000 | 87,000 | 2,500,000 | 292,200 | 41,300,000 | 454,600 |
| White men | 8.8% | 675,000 | 203,900 | 4.9% | 70,800 | 2.3% | 325,000 | 47,200 | 3.1% | 112,600 | 37.8% | 329,600 | |
| White women | 6.6% | 445,000 | 186,500 | 3.0% | 61,600 | 3.2% | 365,000 | 74,700 | 1.8% | 148,600 | 33.3% | 372,200 | |
| Black men | 9.6% | 70,000 | 22,900 | 5.1% | 7,500 | 3.9% | 45,000 | 7,500 | 4.2% | 11,300 | 45.9% | 47,400 | |
| Black women | 9.0% | 65,000 | 23,100 | 2.2% | 8,000 | 4.1% | 60,000 | 10,000 | 4.2% | 14,900 | 45.9% | 52,400 | |
| Mexican American men | 5.4% | – | – | 1.1% | 2.1% | 2.1% | 26.1% | ||||||
| Mexican American women | 6.3% | – | – | 3.8% | 1.4% | 32.5% | |||||||
| Hispanic or Latino | 5.7% | – | – | 3.7% | 8.8% | ||||||||
| Asian | 4.3% | – | – | 2.6% | 6.9% | ||||||||
| American Indian | 5.6% | – | – | – | 10.5% | ||||||||
In the United States, CHD causes about 610,000 new and 325,000 recurrent MIs, per year.21 First MI occurs, on average, at 70.3 years of age for women and 64.5 years of age for men. Framingham Heart Study indicates that women develop CHD an average 10 years later than men and develop events such as MI 20 years later or more.21 In premenopausal women, annual CHD event rates are less than 1%, but there is a two- to three-fold increase after menopause. The first coronary presentation for women is more likely to be angina; in men, it is more likely to be MI. According to the AHA, the age-adjusted annual CHD rates per 1000 population between ages 45 and 64 years are 12.5 in white men, 10.6 in black men, 4.0 in white women, and 5.1 in black women.21
Unrecognized MIs are common, numbering at least one in three MIs in the Framingham Study.41 Half the unrecognized MIs are silent, and the rest are atypical so that neither the patient nor the physician entertains the possibility. More than half of these people eventually develop some overt clinical manifestations of CHD and hence come under medical care. Angina is less frequent in individuals with unrecognized MI than it is in those with recognized symptomatic MI, either before or after the infarction occurs. Despite the apparent mild nature of unrecognized MI, the risk of subsequent mortality is nearly the same as in patients with recognized infarction.41 Men with diabetes and persons with hypertension of both genders are particularly susceptible to unrecognized MIs.41
The lifetime risk of developing CHD after age 40 years is 49% in men and 32% in women. Even at age 70 years, the risk is 35% for men and 24% for women.42
In patients who survive the acute stage of an MI, the morbidity and mortality ranges from 1.5 to 15 times that of the general population, depending on the person’s gender and clinical outcome, with an average number of life years lost estimated at 14 years. The rates of reinfarction, sudden death, angina pectoris, cardiac failure, and stroke are all substantial. The relative and absolute risks of these events after MI are as great in women as in men. In the initial year after a recognized MI after age 40 years, 18% of men and 23% of women die. In the first 5 years after MI, 16% of men and 22% of women between 40 and 69 years of age will have a recurrent infarction. About 7% of men and 12% of women between 40 and 69 years of age and 22% of men and 25% of women over age 70 years of age are disabled with CHF; 4% of men and 6% of women between 40 and 69 years as well as 6% of men and 11% of women older than age 70 years will have a stroke.21 Sudden death will occur in 7% of men and 6% of women, a rate that is four- to six-fold higher than the general population.43
CHD is the single leading cause of death in adults in the United States, accounting for one in five deaths.43 About every 25 seconds, an American will sustain a coronary event, and about every minute, someone will die from one. About 37% of persons who have a coronary attack die. There are about 445,700 coronary deaths every year (see Table 2–6).21
Age, gender, ethnicity, and geographic origin are key correlates of CHD mortality. CHD mortality increases with age, and CHD is also a prominent cause of death in adults at the peak of their productive lives. CHD is the leading cause of death in both men and women and in every racial or ethnic group (except Asian American women). Overall, the CHD death rate is almost three times higher in men than it is in women at ages 25 to 34 years, but this ratio declines to 1.6 by ages 75 to 84 years. In 2005, the overall CHD death rate was 144.4 per 100,000 in the population. CHD death rates are higher in blacks (213.9 for black men and 140.9 for black women) compared with whites (187.7 for white men and 110.0 for white women).21 CHD mortality is not as high among the Asian, American Indian, and Hispanic populations (rates per 100,000 of 81.0, 96.2, and 118, respectively) as it is among blacks and whites.
There has been a progressive decline in the age-adjusted CHD death rate in the United States over the past 5 decades. Framingham data indicate an overall 59% decline in CHD death rates between 1950 and 1999.44 From 1995 to 2005, CHD death rates fell 34.3%, and actual CHD deaths declined by 19.4%.21
Sudden, unexpected, out-of-hospital coronary death that occurs too rapidly to allow arrival alive at the hospital accounts for half of all coronary fatalities. Age, gender, and time since MI are important determinants of sudden death. The proportion of coronary deaths that are sudden is lower in women than it is in men and is lower in elderly men than it is in the young. Sudden cardiac death rates have declined by about 49% over a 50-year observation period (1950-1999) in the Framingham Study.44
Stroke
Age, elevated blood pressure, smoking, diabetes mellitus, electrocardiographic (ECG) left ventricular hypertrophy, and atrial fibrillation are the major risk factors for stroke.26,45 A stroke risk score has been developed to estimate the risk of stroke using the experience of the Framingham cohort.46
It is estimated that 15 million people have a stroke each year, and 5 million incur a permanent disability as a result. There are 5.7 million stroke deaths worldwide each year.20 Strokes accounted for loss of 46.6 million DALYs worldwide in 2004 (see Table 2–1). Every year, there are about 9 million new strokes and 30.5 million prevalent cases worldwide (see Table 2–2).47,48
Stroke mortality has declined in the HIC over the past 2 decades. Data from the MONICA Study demonstrate a modest contribution of reduction in risk factors such as hypertension to the decline in stroke mortality in women but not in men.49 Recent studies collaborating with WHO found a 10-fold range of mortality and DALY among countries. National prevalence of increased mean systolic blood pressure, low body mass index (BMI), and per capita income were predictors of stroke mortality.50
Global mortality attributable to cerebrovascular disease in the next 2 decades will parallel the CHD trends noted earlier with stroke death increasing from 5.7 million in 2005 to 6.5 million in 2015 and to 7.8 million in 2030.51
In the US adult population, 2.6%, or 6.5 million people—have prevalent cerebrovascular disease (stroke or transient ischemic attack; see Table 2–6). Roughly 13 million people have silent strokes on MRI scans of the brain. Prevalence rises from 2% in men at 45 to 54 years to 12.5% for men age 75 and older and from 1% to 10.7% in the corresponding age groups in women. There is geographic and racial heterogeneity in the burden caused by stroke. There is a higher prevalence of stroke in 10 southeastern states relative to the non-southeastern states and District of Columbia, and the prevalence is higher in blacks compared with whites.43 The estimated direct and indirect costs associated with stroke are about $68.9 billion for the year 2009.21
In the United States, someone has a stroke every 40 seconds. Every year, there are approximately 795,000 strokes, of which 610,000 are first events.21 In the Framingham Study, the chance of having a stroke before age 70 years was 5% for both genders. Overall in the United States, the age-adjusted stroke incidence rates (per 1000) for first-ever strokes are 3.6 for white men, 2.3 for white women, 6.6 for black men, and 4.9 for black women.21 Thus, blacks have almost twice the risk of first-ever stroke compared with whites. Although men have 1.25 times the stroke incidence rate compared with women overall, there are about 55,000 more women than men with prevalent stroke because of the greater proportion of women in the older age groups.21
Of the incident stroke events in the United States, 87% are ischemic strokes, and 13% are hemorrhagic (10% are intracerebral hemorrhages, and 3% are subarachnoid hemorrhage).21 Among the 54% classified as definite thrombotic brain infarctions, 38% were classified as lacunar, with more than twice as many found in blacks than whites.43
The lifetime risk of developing stroke after age 55 years is one in six for men and one in five for women, exceeding the lifetime risk of developing Alzheimer’s disease.52
The time course of functional recovery is strongly related to the initial stroke severity. Of survivors of an initial event, 50% to 70% return to functional independence, but 15% to 30% become permanently dependent. Institutional care is required by 20% at 3 months after onset. However, stroke attacks have become less severe in recent years.21
Cerebrovascular disease is the third leading cause of death in the United States and is responsible for 143,600 deaths each year (see Table 2-6).21 On average, every 3 to 4 minutes, someone in the United States dies of a stroke, accounting for one in every 17 deaths. For patients older than 65 years of age, the proportion of strokes that results in death within 30 days is about 12.6%.
Mortality varies according to stroke type. Approximately 8 to 12% of ischemic stroke patients die within 30 days compared with 37% of patients with hemorrhagic strokes. Age, ethnicity, and geographic region are other key determinants of stroke mortality. Overall, stroke mortality is higher in elderly individuals and in blacks. The 2005 overall death rate for stroke was 46.6. Rates of stroke death were 70.5 for black men, 44.7 for white men, 60.7 for black women, and 44.0 for white women. Stroke death rates are lower in other ethnicities (33.5 [women] -38.0 [men] for Hispanics, 31.3 [men] -37.1 [women] for American Indians and Alaska Natives, and 36.3 [women] -41.5 [men] for Asian and Pacific Islanders in 2005).21 Stroke death rates are higher in regions in the southeastern United States, referred to as the stroke belt. In adults ages 25 to 44 years, the mortality rate is three times greater in blacks and American Indians and Alaskan Natives than it is in whites, largely as a result of the higher prevalence and increased severity of hypertension.
In the United States, the age-adjusted death rate for stroke has also declined by more than 50% over the past 4 decades, although the decline appears to have slowed in the 1990s; the rate of decline was 4 to 6% per year in the 1970s and early 1980s.24 From 1995 to 2005, the stroke death rate decreased 29.7%, and the actual number of stroke deaths declined only 13.5%.43 The overall decline in stroke mortality is remarkable because the population of older persons increased substantially during that time.
Congestive Heart Failure
Advancing age, MI, hypertension, diabetes mellitus, valvular heart disease, and obesity are key risk factors for CHF.53 High blood pressure antedates more than 75% of heart failure.54 A clinical risk score has been formulated to estimate the risk of developing CHF based on several of these risk factors.55
CHF is clearly a major clinical and public health problem. The exact magnitude of the problem is difficult to assess because we lack broad-based population estimates of its prevalence, incidence, and mortality rates. It is estimated that there were nearly 19 million people with heart failure worldwide in 2004.47
It is estimated that the burden of CHF will increase over the next 2 decades in HIC.56 Despite a stable incidence rate, the increasing prevalence may result from a reduction in CHF mortality.57
The AHA estimates that 5.7 million people in the United States have CHF as of 2006 with incidence rates approaching 10 per 1000 after age 65 years (see Table 2–2).21 CHF is reported to be the leading diagnosis for hospitalization of persons older than age 65 years. The estimated health care costs (direct and indirect) for heart failure for 2009 were $37.2 billion.
The population-based estimates from the Framingham Study indicate an increase in prevalence in men and women from 8 per 1000 at age 50 to 59 years to 66 per 1000 at age 80 to 89 years in men and 79 per 1000 in women. The prevalence of heart failure in blacks is reported to be higher than it is in whites. The age-adjusted prevalence of heart failure in whites is 3.1% in men and 1.8% in women, in blacks is 4.2% in men and 4.2% in women, and in Mexican Americans is 2.1% in men and 1.4% in women.21 Hypertension and ECG left ventricular hypertrophy are more common in blacks with CHF, but they have a lower prevalence of CHD and valvular disease.58
At present, epidemiologic population-based assessment of the prevalence of diastolic CHF uses the occurrence of clinically overt heart failure in persons with normal left ventricular systolic function for case ascertainment. Approximately 30% to 50% of patients with CHF are reported to have a normal or nearly normal left ventricular ejection fraction.59-61 In the Framingham Study, women predominated in the diastolic CHF subgroup, with 65% of heart failure occurring in association with a normal left ventricular ejection fraction. In men, 75% of the heart failure cases occurred in those with left ventricular systolic dysfunction.62 The high prevalence and female preponderance in diastolic CHF have been corroborated by numerous other community-based investigations.59-61 Recent data suggest a trend of increasing diastolic CHF prevalence in the community.60
The Framingham Study reported that the incidence of CHF increased steeply with age, approximately doubling with each decade.53 Between the ages of 35 to 64 and 65 to 94 years, the annual incidence rate in men increased from 3 per 1000 to 12 per 1000. In women, the corresponding rates were 2 and 9 per 1000. The higher rate in men at all ages is chiefly attributable to the greater vulnerability of men to CHD. Similar figures for the incidence of CHF have been reported by other cohort studies and investigations examining new cases in other geographic regions worldwide.
The Framingham Study reported that the lifetime risk of CHF is 21% in men and 20% in women.63 Furthermore, lifetime risk of CHF, even in the absence of a myocardial infarction, is 11% in men and 15% in women.63
The annual total-mention death rate for CHF per 1000 population in the year 2005 was higher in blacks (58.7 for women and 81.9 for men) compared with whites (43.2 for women and 62.1 for men).21 In population-based studies, the survival rates of CHF patients are appalling. The overall population rate of expected life-years lost due to CHF is 6.7 years per 1000 in men and 5.1 years per 1000 in women. Geographically, there is about a 10-fold range of reported mortality from CHF with the highest rates reported from the southern stroke belt. The age-adjusted death rates are 25% higher in men than they are in women and are 40% higher in blacks than they are in whites. The lower mortality in women may be related to a greater likelihood of a false-positive diagnosis of CHF, a lower probability of coronary disease as the basis of heart failure, and a higher prevalence of intact left ventricular systolic function.
In the Framingham Study, a number of other conditions were associated with a poor survival in individuals with CHF. Advancing age was associated with increased mortality—27% per decade in men and 61% in women. Valvular heart disease increased the hazard by 68% in men, while in women, diabetes mellitus imposed a 70% higher mortality rate. Additional prognostic factors associated with an adverse outcome include the presence of atrial fibrillation, renal dysfunction, underlying diabetes mellitus, a low BMI, and a low systolic blood pressure. In the Framingham Study, patients with diastolic CHF had an annual mortality rate of 8.7% compared with 18.9% for those with systolic CHF. Compared with age- and gender-matched control subjects, diastolic and systolic CHF were associated with hazard ratios for mortality of 4.1 and 4.3, respectively.62 More recent studies have demonstrated more modest differences60 or no differences59 in the survival of patients with diastolic versus systolic CHF.
Data from several sources suggest that blacks with CHF have a worse prognosis relative to whites even after adjusting for multiple factors. Blacks with CHF also experience more hospital readmissions than whites.58
Atrial Fibrillation
The risk factors for atrial fibrillation include standard CVD risk factors such as advancing age, male gender, increasing BMI, hypertension, diabetes, heart failure, MI, valvular heart disease, and increasing left atrial size.64,65 Other factors associated with atrial fibrillation include alcohol consumption, hyperthyroidism, and reduced lung function.66,67 Recent studies have reported that elevated biomarkers such as C-reactive protein (CRP) and B-type natriuretic peptide concentrations also predict an increased risk,68,69 although whether these biomarkers are causally related to atrial fibrillation remains to be determined. Additionally, increasing evidence suggests that there is a genetic predisposition to atrial fibrillation.70-72 A risk score to predict atrial fibrillation using clinical variables available to office-based clinicians was recently formulated.73
In contrast to HIC, in LMIC, valvular heart disease appears to be the most common predisposing condition.74 However, with the globalization of cardiovascular disease risk factors, hypertension and CHD are increasing in importance in LMIC.
The global burden of atrial fibrillation is unknown because most atrial fibrillation research has been conducted in North America and Western Europe.75 Even within these geographic constraints, the reported studies have been from predominantly white cohorts. Also, our ability to compare the epidemiology of atrial fibrillation in different countries and ethnicities has been limited by study design issues, such as age, case ascertainment, case definition (chronic versus paroxysmal; atrial flutter and atrial fibrillation), duration of follow-up, and frequency of ECG surveillance across studies.75 For instance, the prevalence of atrial fibrillation in a community-based study of Japanese at least 40 years of age with a single-occasion ECG was 1.3%;76 the prevalence of chronic atrial fibrillation in Indo-Asians older than age 50 years retrospectively identified from a chart review of six general practices in England was 0.6%.77 Higher prevalence rates have been described from US cohort studies with routine surveillance of ECGs (see later and review75 for country-specific data).