Chapter 13 HISTORY OF THE BICUSPID AORTIC VALVE ANATOMY, PATHOLOGY, CLASSIFICATION SCHEMES ASSOCIATED CARDIOVASCULAR LESIONS CLINICAL PRESENTATION AND IMAGING ASSOCIATED ABNORMALITIES OF THE AORTIC WALL Aortic Medial Disease and Ascending Aortic Dilation Pathophysiology and Molecular Biology of Bicuspid Aortic Valve Aortopathy LATE AORTIC COMPLICATIONS FOLLOWING BICUSPID AORTIC VALVE REPLACEMENT FAMILIAL BICUSPID AORTIC VALVE AND ASCENDING AORTIC ANEURYSMS SURGICAL TREATMENT OF THE BICUSPID AORTIC VALVE AND ASCENDING AORTA The BAV has been long recognized as an important cause of valvular heart disease. Leonardo da Vinci sketched the bicuspid variant of the aortic valve more than 400 years ago. 1 The clinical and valvular sequelae of the BAV were realized more than 150 years ago.1,2 Osler’s landmark report of 18 cases of BAV in 1886 emphasized the frequent complication of infective endocarditis in this lesion.1,2 The fact that aortic stenosis occurred in BAV as a result of a primary valve pathology rather than rheumatic disease was realized in the 1950s.1,2 Autopsy studies established BAV as the most common congenital anomaly of the heart. The association of congenital BAV with diseases of the aorta was first recorded by Abbott 3 in 1927. In 1984, Larson and Edwards 4 highlighted the relationship between the BAV and aortic root disease, noting a ninefold greater risk of aortic dissection in patients with BAV. 4 The semilunar valves originate from the mesenchymal outgrowths (cardiac cushions) along the ventricular outflow tract of the primary heart tube. Endocardial cushion formation has been studied in many different species, and several molecular signaling pathways have been implicated in the development of the atrioventricular and outflow tract regions, including transforming growth factor-beta (TGF-β), Ras, Wnt/B-catenin, vascular endothelial growth factor (VEGF), and NOTCH signaling.5,6 The pathogenesis of BAV is unknown. In a Syrian hamster model of BAV, fusion of the right and the left valve cushions appears to be a key factor, and BAV is not the consequence of improper development of the conotruncal ridges, conotruncal malseptation, or valve cushion agenesis. 7 Anomalous behavior of cells derived from the neural crest has been implicated as a possible etiology because BAV is often associated with congenital malformations of the aortic arch and other neural crest-derived systems.1,8,9 A primary molecular abnormality of the extracellular matrix may trigger abnormal valvulogenesis, given that matrix proteins help direct cell differentiation and cusp formation during valvulogenesis. 10 Endothelial nitric oxide (eNOS) signaling may be important in BAV pathogenesis and possibly in the associated aortic disease.11,12 Different molecular and biologic pathways may be responsible for leaflet orientation among animal species with BAV ( Figure 13-1). In knockout mice, fused right and noncoronary leaflet BAVs result from a defective development of the cardiac outflow tract endocardial cushions that may rely on a nitric oxide–dependent epithelial-to-mesenchymal transformation. 9 In inbred Syrian hamsters, fused right and left coronary leaflet BAVs are due to an extrafusion of the septal and parietal outflow tract ridges likely caused by a distorted behavior of neural crest cells. 9 FIGURE 13-1 Aortic valve morphology in animal models of bicuspid aortic valve. The GATA family of zinc finger proteins may be important in cardiac development. Gata5 expression is restricted to endocardial cushions, and targeted inactivation of Gata5 in mice leads to development of BAV, involving several signaling pathways (including Notch). 13 The ubiquitin fusion degradation 1–like gene is expressed in the developing embryonic outflow tract and is downregulated in BAV tissue. 14 NOTCH1 encodes for a transmembrane protein that activates a signaling pathway important in cardiac embryogenesis, including the aortic and pulmonary valve and aorta. NOTCH1 mutations (9q34.3) have been found in a small number of families with BAV and ascending aortic aneurysms. 5 The anatomy of the BAV includes unequal cusp size (due to fusion of two cusps leading to one larger cusp), a raphe (usually in the center of the larger of the two cusps), and smooth cusp margins ( Figure 13-2). The raphe or fibrous ridge is the site of congenital fusion of the two components of the conjoined cusps and is identifiable in most cases. 15 There is a wide spectrum of BAV, from completely missing one commissure—leading to two cusps, sinuses, and commissures only—to an underdevelopment of one or two commissures and the adjacent cusps, which occurs in the majority of cases with one or two raphes. 16 Fusion may occur between any of the leaflets, most commonly between the right and left coronary leaflets (70% to 86%), although also between the right and noncoronary leaflets (12%), and least commonly between the left and noncoronary leaflets (3%). 1 With right and left coronary leaflet fusion, the leaflets are oriented right and left and the true commissures are oriented in an anterior and posterior manner. 17 The coronary arteries tend to arise from the front of the cusps, in which a raphe is present. BAVs without any redundant tissue tend to develop stenosis, whereas valves with more redundant tissue usually develop valvular incompetence. 1 FIGURE 13-2 Bicuspid aortic valve anatomy. Various classification schemes have been used to characterize the BAV16,18 ( Figure 13-3). A review of echocardiograms of 1135 children with BAV revealed that in 70% of cases, right coronary and left coronary leaflet fusion was the most common morphologic variant (pattern A). 19 Pattern B, or the fusion of the right coronary and noncoronary leaflet, was more likely to be associated with aortic stenosis or regurgitation in this pediatric series. Pattern C (fusion of the left coronary and noncoronary cusps) was the least common morphologic variant. In a surgical pathology study of 542 cases of BAV, 86% were noted to have pattern A, 12% pattern B, and 3% pattern C. 15 FIGURE 13-3 Classification schemes of bicuspid aortic valves. The valve orientation of a BAV may be predictive of clinical outcomes.19,20 Fusion of the right and noncoronary valve leaflets has been associated with more rapid progression of aortic stenosis and regurgitation than fusion of the right and left coronary leaflets, 20 although later population studies did not demonstrate an association of leaflet orientation with valvular degeneration.21,22 In addition, leaflet orientation in BAV may also be predictive of aortic elastic properties. 23 Calcium deposition and the development of fibrosis of the BAV increases with age and is largely confined to the raphe and base of the cusp2,24 ( Figure 13-4). The calcification process occurring in patients with BAV is similar to that in those with trileaflet aortic valve but occurs at an accelerated rate and includes lipid deposition, neoangiogenesis, and inflammatory cell infiltration.1,25 BAVs demonstrate folding or wrinkling of the valve tissue, increased doming of the leaflets during the cardiac cycle, and abnormal currents of turbulence, even when the leaflets are not stenotic. 26 These factors may increase susceptibility to BAV degeneration. As a result of multiple mechanisms, most patients with BAV require valve surgery during their lifetimes.24,27 The prevalence of BAV is approximately 1% of the population with a male/female ratio of between 2:1 and 3:1.1,2,28–31 ( Table 13-1). In the largest reported necropsy series involving 21,417 consecutive cases, 293 subjects were noted to have BAV, for a prevalence rate of 1.37%. 31 TABLE 13-1 Prevalence of Bicuspid Aortic Valve (BAV) in Reported Necropsy Studies Adapted from Basso C, Boschello M, Perrone C, et al. An echocardiographic survey of primary school children for bicuspid aortic valve. Am J Cardiol 2004;93:661–3. See the paper by Basso et al for the full citations for the studies listed in this table. Echocardiography screening has improved understanding of the prevalence of BAV in the general population. In an echocardiography study of 817 asymptomatic children, a BAV was found in 4 of the 817 children (0.5%), 3 of 4 being found in males. 29 In an echocardiography study of 1075 neonates, the prevalence of BAV was 4.6 per 1000 live births (7.1 per 1000 males and 1.9 per 1000 females). 32 Of 20,946 military recruits in Italy, the prevalence of BAV was 0.8%. 33 This may be an underestimation, as only those with an abnormal history, physical examination, or ECG underwent screening echocardiograms. 33 Certain patients have a much higher prevalence of BAV than the general population, including approximately 50% of patients with coarctation of the aorta 1 and 30% of females with Turner syndrome.34,35 Case reports describing the familial clustering of BAV and reports of BAVs in monozygotic twins underscored the genetic predisposition for BAV.36–39 In a study of 41 families with surgically proven BAV in one member, 15% of the families were noted to have more than one member with BAV. 39 In families in which more than one member had aortic valve disease, 24% of relatives had evidence of aortic valve disease, likely secondary to a BAV. 40 Echocardiographic studies in families of patients with BAV report the prevalence of BAV in first-degree relatives of an individual with BAV to be about 9%.41,42 With the use of variance component analysis, the heritability (h2) of BAV was calculated to be 89%. 42 The inheritance of BAV is consistent with an autosomal dominant pattern with reduced penetrance.41,42 Diverse genes with dissimilar inheritance patterns in families are considered responsible. 42 The specific gene loci or products, whether structural proteins or ones with vital roles in cardiac development, that are responsible for the development of BAVs have yet to be discovered. Nongenetic factors also play an important role in this development. 43 In animal models with BAV, potential mechanisms and pathways have been reported in eNOS, NKX2.5, and NOTCH signaling. 5 Human studies have demonstrated the genetic influences on left-sided outflow lesions, including hypoplastic left heart syndrome (HLHS) and BAV. 5 An increased prevalence of BAV in probands and family members of patients with HLHS is recognized, and linkage analysis demonstrates that some cases of HLHS and BAV are genetically related.44,45 In a study evaluating the first-degree relatives of pediatric patients with left ventricular outflow tract obstruction, 20% of families had another individual with a cardiac anomaly, most commonly a BAV. 46 DiGeorge and velocardiofacial syndromes, involving deletions within chromosome 22q11.2, have been associated with concomitant BAV. Gene network analysis techniques have identified AXIN1-PDIA2 and endoglin haplotypes associated with BAV. 47 Anderson syndrome, due to a mutation in KCNJ2 and associated with abnormal potassium signaling, is associated with an increased prevalence of BAV. 48 NOTCH1 mutations have been found in a small number of families with nonsyndromic BAV or with BAV and ascending aortic aneurysms 5 (see later section Familial Bicuspid Aortic Valve and Ascending Aortic Aneurysm). Because of the familial nature of BAV, screening of first-degree relatives for BAV has been recommended in the 2008 American College of Cardiology/American Heart Association (ACC/AHA) guidelines for the management of adults with congenital heart disease. 49 In most instances, BAV is an isolated cardiovascular finding. However, BAV may coexist with a number of other congenital cardiovascular defects or syndromes ( Table 13-2). The presence of a BAV may account for significant morbidity associated with these syndromes and should trigger an evaluation for related cardiovascular disorders. Conversely, the presence of any of the lesions discussed here should also prompt further search for the presence of BAV. TABLE 13-2 Bicuspid Aortic Valve (BAV) and Associated Cardiovascular Conditions Coarctation of the aorta (CoA) can be either “simple” (isolated defect) or “complex” (associated with other intracardiac or extracardiac defects). A BAV occurs in 25-75% of complex aortic coarctation. The BAV accompanying CoA has been described as “equally bicuspid” with two symmetric sinuses of Valsalva. 1 Morphologic analysis of the BAV has demonstrated an increased frequency of fusion of the left and right coronary cusps in the presence of CoA. 50 Identification of BAV in patients with coarctation is vital because its presence confers a substantially increased risk for aortic aneurysm and dissection3,25,51,52 ( Figure 13-5). In addition, valvular complications from the BAV such as aortic stenosis and regurgitation are more prevalent in subjects with both CoA and BAV. 51 FIGURE 13-5 Cardiac magnetic resonance imaging (CMR) and computed tomography (CT) of the aorta in patients with a bicuspid aortic valve and coarctation of the aorta (CoA). Individuals with BAV require long-term follow-up not only of the coarctation repair but also of the BAV and ascending aorta.51–53 In one large series of patients with surgical coarctation repair, 41% of those who needed reoperation did so primarily for valvular indications. 53 Such patients should undergo meticulous follow-up with routine echocardiographic and radiographic assessment. Turner syndrome is characterized by complete or partial absence of one X chromosome. About 50% of cases are due to the 45,XO karyotype, and the remainder are due to 45,XO/XX mosaicism or other X chromosomal abnormalities. 35 Cardiovascular defects occur in up to 75% of patients with Turner syndrome. 54 BAV is present in about 30% of cases,35,54,55 and associated defects include ascending aortic dilation, aortic coarctation, pseudocoarctation and elongated aortic arch.54–56 In patients with Turner syndrome, BAV is most commonly due to fusion of right and left coronary cusps. The presence of BAV is associated with larger aortic dimensions at the annulus, sinuses, sinotubular junction, and ascending aorta. 35 Patients with Turner syndrome may have a shortened life expectancy, death most commonly occurring from cardiovascular causes.1,56 Because patients with Turner syndrome have short stature, ascending aortic dimensions may be significantly dilated relative to body surface area. Because of the small stature of such patients, prophylactic aortic surgery is recommended at smaller aortic dimensions, and the aortic size should be indexed to body surface area.56,57 Screening for BAV and other cardiovascular abnormalities as well as serial echocardiographic and radiographic follow-up evaluations is necessary in patients with Turner syndrome. Other congenital heart diseases and syndromes commonly associated with BAV are listed in Table 13-2. 1 Congenital coronary anomalies have been described in association with BAV, with left coronary artery dominance in 24% to 57%.1,14,58 Additionally, patients with BAV have shorter left main coronary arteries than patients with a trileaflet aortic valve. 58 Isolated congenital coronary artery anomalies have also been reported with BAV.1,59 These features should lead one to consider evaluation of the coronary anatomy prior to elective valve surgery with either catheter-based or computed tomography (CT) coronary angiography to prevent coronary injury and provide for adequate intraoperative myocardial protection. The clinical examination of the patient with BAV is variable and depends on the function of the valve and any associated lesions. The majority of young patients with isolated BAV are asymptomatic and are diagnosed incidentally when a systolic ejection sound or murmur is noted or on echocardiography. A functionally normal BAV has an ejection sound or “click” and is often followed by an early peaking systolic flow murmur. The ejection sound is a reflection of the sudden cephalad movement of the dome-shaped bicuspid valve in systole and generally correlates with valve leaflet mobility. 1 In the setting of progressive aortic stenosis, the ejection murmur becomes harsher and later peaking. It is accompanied by a displaced and sustained left ventricular (LV) impulse and decreased arterial pulses. The ejection sound diminishes as the valve cusps become more immobile. In the setting of an incompetent BAV, the findings vary with the severity of lesion. An ejection sound typically is present with mild to moderate aortic regurgitation (AR) and absent when regurgitation is severe.1,60 With significant AR, a typical early diastolic decrescendo murmur is best heard at the left lower sternal border. When the murmur of AR is heard loudest at the right midsternal border, concern should arise about the presence of a dilated ascending aorta complicating the BAV. The chest radiograph may provide significant clues as to the presence of BAV and any sequelae related to valvular complications or associated vascular lesions. However, the findings may be entirely unremarkable. Aortic valve calcification may be detected on the plain film and is best seen on the lateral projection. 61 A complete or partial ring with or without a calcified central raphe characterizes the distinctive pattern of calcification of the BAV. In general, LV size remains normal unless advanced heart failure is present.60,61 In the setting of chronically regurgitant BAV, an enlarged cardiac silhouette may be noted. The chest radiograph is notoriously inadequate to detect dilation of the aortic root. On occasion, a dilated aortic shadow may be present. When aortic coarctation is associated with BAV, rib notching and convexity of the proximal descending aorta are often seen. 60 In light of the clinical significance of BAV, echocardiographic identification and characterization are imperative. A number of distinct features of the BAV seen on transthoracic echocardiography (TTE) assist in making this diagnosis1,18 ( Table 13-3). Care must be taken to assess the valve in both systole and diastole (see Chapter 6). In patients with a prominent raphe, the valve may appear trileaflet in diastole, but the distinct elliptical or “football-shaped” orifice is visualized in systole, indicating that the raphe is not a functional commissure ( Figure 13-6A and 6B). Table 13-3 Echocardiographic Features of the Bicuspid Aortic Valve Systolic doming Eccentric valve closure Leaflet redundancy Presence of raphe (often calcified) Elliptical (“football”-shaped) systolic orifice Distinct opening pattern: opens from the center and separates at the commissures in a curvilinear fashion Eccentric jets of aortic regurgitation Dilated ascending aorta FIGURE 13-6 Transthoracic echocardiography of bicuspid aortic valve. The leaflets of a BAV are often thickened and calcified out of proportion to the patient’s age. Prominent systolic doming of the leaflets and eccentric valve closure are often noted on the parasternal long-axis view1,18 ( Figure 13-6C and 6E). It is noteworthy that up to 25% of BAVs may not demonstrate eccentric closure, and conversely, trileaflet valves may infrequently have eccentric closure. The presence of leaflet redundancy on parasternal and apical long-axis views should suggest the presence of a BAV. Unexplained eccentric jets of AR may also suggest an underlying BAV. When poor acoustic windows or technical limitations prohibit adequate leaflet visualization, the opening pattern of the valve may aid in the diagnosis. The BAV tends to open from the center and separate at the commissures in a curvilinear fashion, like a rope going slack from the center, 62 whereas the leaflets of a normal aortic valve maintain a straighter shape in diastole, pivoting from their point of annular insertion. In the diagnosis of BAV, TTE has a sensitivity of 78% to 92% and a specificity of 96%,18,63 but the accuracy depends on image quality, valve calcification, and the experience of the interpreter. Heavy calcification limits the ability to discern leaflet number accurately. A prominent raphe may often give the appearance of a third coaptation line, suggesting a trileaflet valve. Conversely, the aortic valve may appear bicuspid when one of the cusps is diminutive. 62 Ascending aortic enlargement should trigger a careful evaluation for a BAV54,64 ( Figure 13-7). Because aortic dilation is frequently largest in the ascending aorta, the entire aspect of the aortic root and proximal ascending aorta should be imaged to insure accurate assessment of the aortic dimensions.1,57,65 FIGURE 13-7 Aortic root aneurysm complicating a “functionally normal” bicuspid aortic valve. Once BAV is detected, the echocardiogram should include a formal assessment for valvular complications. Aortic stenosis tends to occur at a younger age than trileaflet valve, particularly when the aortic cusps are asymmetric or there is fusion of the right and left cusps. 24 Because of the eccentric nature of the systolic jet, interrogation of the jet via the right parasternal window may yield the highest gradients. Patients with BAV often have larger LV outflow tracts. Therefore, use of the continuity equation may yield larger calculated valve areas, potentially underestimating the hemodynamic severity. The use of serial gradients and velocity time integral ratios may more accurately reflect the hemodynamic burden in these patients. 66 AR may be the main clinical manifestation of BAV in the adolescent or young adult. The jets of BAV AR may be highly eccentric, making severity more difficult to assess. Multiple mechanisms may lead to AR in BAV (see Aortic Regurgitation). When a BAV is present, the echocardiographic examination should include a routine evaluation for the presence of coexisting cardiovascular lesions (see Table 13-2). Care should be taken to assess for the presence of VSD, aortic coarctation, and aortic root and ascending aortic pathology (sinus of Valsalva aneurysm, supravalvular aortic stenosis, aneurysm, aortic dissection), and LV outflow tract abnormalities. The standard examination should include an interrogation of the distal aortic arch via suprasternal notch views to assess for the presence of coarctation. In a subset of patients the morphology of the aortic valve cannot be accurately determined by transthoracic echocardiogram. Transesophageal echocardiography (TEE) is useful in such cases for diagnosis of BAV. Multiplane TEE is highly accurate at detecting BAV, with reported sensitivity of 87% and specificity of 91% 67 ( Figure 13-8). The sensitivity of TEE approaches 100% when little valvular calcification is present. In the presence of moderate to severe valvular calcification, sensitivity is lower. 68 Cardiovascular magnetic resonance (CMR) and CT imaging represent vital noninvasive modalities in addition to standard echocardiography in the diagnosis and management of BAV. CT has a high sensitivity (94%) and specificity (100%) for detecting BAVs. 69 CMR is also highly accurate in diagnosing BAV, with a sensitivity of 100% and specificity of 95% 70 ( Figure 13-9). FIGURE 13-9 Cardiac magnetic resonance angiography demonstrating a bicuspid aortic valve. In a study comparing CT, CMR, and TTE in patients undergoing valve surgery, both CT and CMR were highly accurate for identifying aortic valve morphology. 71 Sensitivities, specificities, and positive and negative predictive values for aortic valve morphology assessment (trileaflet versus bicuspid) were: 97%, 95%, 98%, and 94% for CT angiography; 98%, 96%, 98%, and 95% for CMR; and 98%, 88%, 95%, and 96% for TTE. 71 CT has been shown to accurately identify BAV and assess aortic valve area via planimetry when compared with CMR, TEE, and TTE.72,73 CMR correlates well with both echocardiographic and invasive catheter-based techniques for evaluation of stenotic and regurgitant valvular lesions. 72 CMR may also accurately assess BAV morphology on the basis of the orientation of leaflets and raphes. 74 Complications will develop in the majority of patients with BAV during their lifetimes,1,21,22,24 including aortic valve dysfunction, endocarditis, and aortic aneurysm or dissection. For some patients the BAV functions nearly normally for much of their lives, whereas for others, a valve disorder or aortic complication occurs early in life21,22 ( Table 13-4). In a population-based study of 212 initially asymptomatic patients with minimally dysfunctional BAV, survival values were 97% ± 1% and 90% ± 3%, 10 and 20 years after diagnosis, respectively, and were identical to expected survival values in the age- and sex-matched population ( Figure 13-10). 21 However, the 20-year rate for surgical events (aortic valve or aorta) was 27% ± 4% and the rate for any cardiovascular event was 42% ± 5%. 21 As expected, cardiac events were more common in those with increasing age (>30 years) and moderate to severe valvular dysfunction. TABLE 13-4 Late Outcomes in Adults with Bicuspid Aortic Valve (BAV) Disease AS, aortic stenosis; AR, aortic regurgitation. *Data from Michelena HI, Desjardins VA, Avierinos JF, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008;117:2776–84. Cardiovascular medical events = cardiac death, congestive heart failure, new cardiovascular symptoms (dyspnea, syncope, anginal pain), stroke, and endocarditis. Surgical events = aortic valve surgery (aortic valve replacement, repair, or valvotomy) and surgery of the thoracic aorta (for aneurysms, dissection, or coarctation). †Data from Tzemos N, Therrien J, Yip J, et al. Outcomes in adults with bicuspid aortic valves. JAMA 2008;300:1317–25. Primary cardiac events = surgery on the aortic valve or ascending aorta, percutaneous aortic valvotomy, aortic complications (dissection or aneurysm development), congestive heart failure requiring hospital admission or cardiac death. Adapted with permission from Siu SA, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol 2010;55:2789–2800. FIGURE 13-10 Survival in asymptomatic adults with bicuspid aortic valve with no significant aortic valve dysfunction. In another study of the natural history of BAV, survival rates in a cohort of 642 adults in a referral-based population were not significantly different from those in the general population (see Table 13-4). 22 Vascular risk factors such as hypertension, cigarette smoking, and hyperlipidemia were associated with worse outcomes among patients with BAV. 21 The age of the patient and presence of valvular dysfunction and aortic aneurysm also predicted adverse clinical outcomes21,22,75 ( Figure 13-11). FIGURE 13-11 Frequency of adverse cardiac events in adults with bicuspid aortic valve disease stratified according to risk profile.
The Bicuspid Aortic Valve and Associated Aortic Disease
History of the Bicuspid Aortic Valve
Embryology
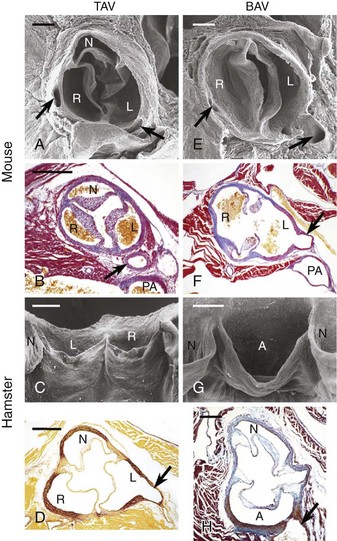
Trileaflet aortic valves (TAVs) (A to D) and bicuspid aortic valves (BAVs) (E to H) in endothelium nitric oxide synthase knockout (eNOS−/–) mice (A, B, E, F) and inbred hamsters (C, D, G, H). Scanning electron micrographs, cranial (A, E) and frontal (C, G) views. In C and G, the specimens were opened through the noncoronary aortic sinus to show the anterior aspect of the valve. Transverse sections stained with Mallory trichrome (B, F), Masson-Goldner trichrome (H), and orcein-picrofuchsin (D) stains. The arrows point to the coronary arteries. In the mouse BAVs, R indicates the right aortic sinus supporting the fused right and noncoronary leaflets. Bars = 200 µm (A, B, E, F) and 400 µm (C, D, G, H). A, Aortic sinus supporting the fused right and left coronary leaflets; L, left aortic sinus; N, noncoronary sinus; PA, pulmonary artery; R, right aortic sinus. (Reproduced with permission from Fernandez B, Duran AC, Fernandez-Gallego T, et al. Bicuspid aortic valves with different spatial orientations of the leaflets are distinct etiological entities. J Am Coll Cardiol 2009;54:2312–18.)
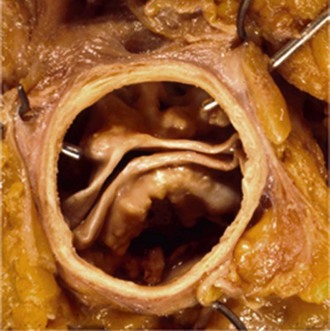
Anatomy, Pathology, and Classification Schemes
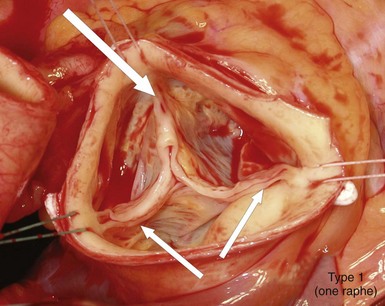
Intraoperative picture of a bicuspid aortic valve type 1 (left-right cusp fusion) with one completely developed noncoronary cusp, two completely developed commissures (small arrows), and one raphe between the underdeveloped left and right coronary cusps extending to the corresponding malformed commissures (large arrow), with hemodynamic signs of regurgitation due to prolapse of the conjoint cusps. (Reproduced with permission from Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007;133:1226–33.)
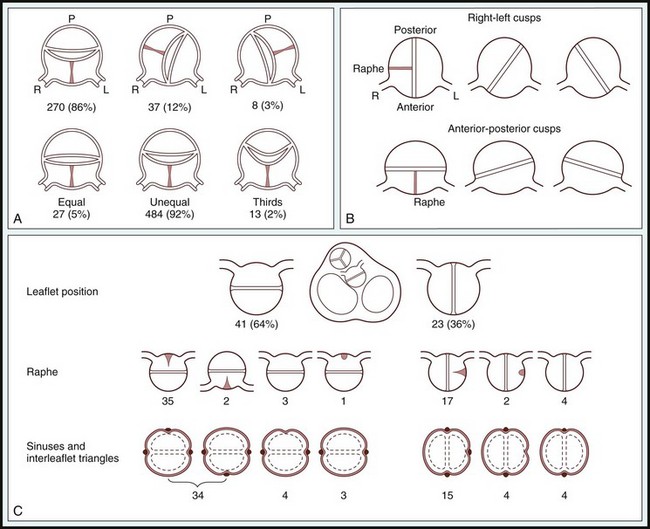
A, Scheme offered by Sabet et al. Top, Relative positions of raphe and conjoined cusp in 315 bicuspid aortic valves. Bottom, Relative cusp sizes in 524 valves (data not available in 18 of 542 cases). L, Left; P, posterior; R, right. B, Classification from Roberts, 2 based on 85 autopsy cases. C, Angelini et al (Angelini A, Ho SY, Anderson RH, et al. The morphology of the normal aortic valve as compared with the aortic valve having two leaflets. J Thorac Cardiovasc Surg 1989;98:362-367.) The positions of the raphe and cusps, the relative sizes of cusps, as well as the number of sinuses and interleaflet triangles as described from the left ventricular outflow were main but not uniform determinants for classifying bicuspid aortic valves. (From Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007;133:1226–33.)
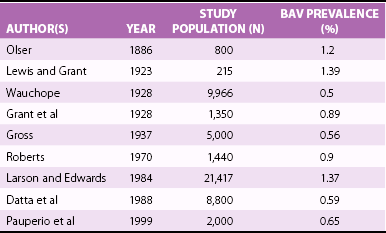
Prevalence
Genetics
Associated Cardiovascular Lesions
CONDITION
INCIDENCE OF BAV (%)
COMMENTS
Coarctation of the aorta (CoA)
50
BAV confers increased risk of aortic complications
Turner syndrome
30
Most frequent cardiac abnormality
Right-left cusp fusion most common
Supravalvular aortic stenosis
30
Usually part of William syndrome
BAV associated with higher risk of reoperation
Subvalvular aortic stenosis
23
May result in significant aortic regurgitation
Patent ductus arteriosus
Unknown
Usually diagnosed in childhood/infancy
Sinus of Valsalva aneurysm
15-20
Frequently asymptomatic
Most commonly involves right coronary sinus
Ventricular septal defect
30
May result in significant aortic regurgitation
Shone complex
60-85
Series of left-sided obstructive lesions (supravalvular mitral ring, parachute mitral valve, subaortic stenosis, CoA)
Ascending aortic dilation
Common
BAV is one of the most common associates of a dilated ascending aorta
Aortic aneurysm syndromes:
Loeys-Dietz syndrome
2.5-17
TGFBR1 or TGFBR2 mutations
Familial thoracic aortic aneurysm syndrome
3
ACTA2 mutations
Coarctation of the Aorta
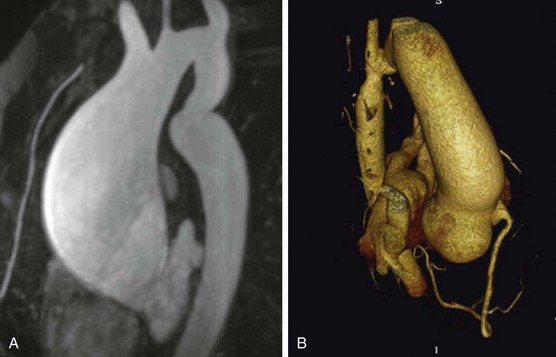
A, CMR of ascending aortic aneurysm in patient with unrepaired mild CoA who died suddenly from an aortic wall rupture. B, Three-dimensional image on multidetector CT scan of posterior sinus of Valsalva aneurysm in patient with stented CoA. (From Oliver JM, Alonso-Gonzalez R, Gonzalez AE, et al. Risk of aortic root or ascending aorta complications in patients with bicuspid aortic valve with and without coarctation of the aorta. Am J Cardiol 2009;104:1001–6.)
Turner Syndrome
Associated Congenital Heart Malformations
Coronary Artery Anomalies
Clinical Presentation and Imaging
Physical Examination
Chest Radiography
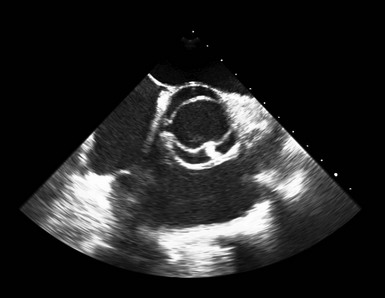
Transthoracic Echocardiography
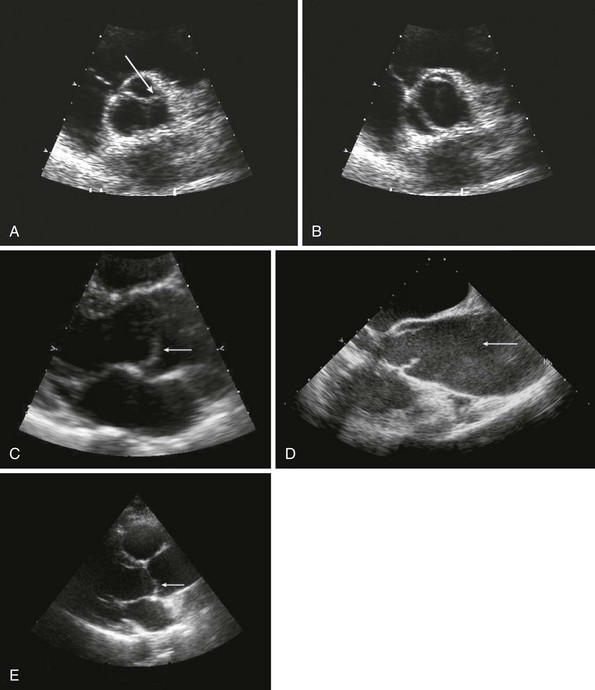
A, Short-axis view of the bicuspid aortic valve in diastole, demonstrating asymmetrical sinuses and a raphe (arrow). B, Bicuspid aortic valve in systole with elliptical opening pattern. C, Long axis view showing prominent systolic doming of the aortic valve leaflets (arrow). D, Dilated ascending aorta (arrow), E, Eccentric closure of the aortic valve (arrow denotes coaptation point).
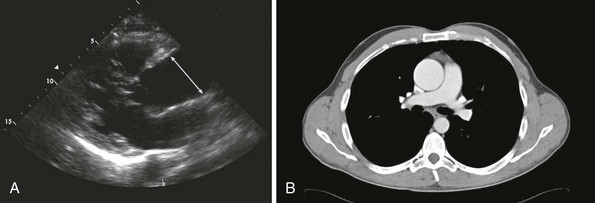
A, Transthoracic echocardiogram of a 4.9-cm ascending aorta (line denotes ascending aortic aneurysm). B, Corresponding computed tomography (CT) scan of the aortic root aneurysm.
Valvular Complications
Associated Lesions
Transesophageal Echocardiography
Other Imaging Approaches
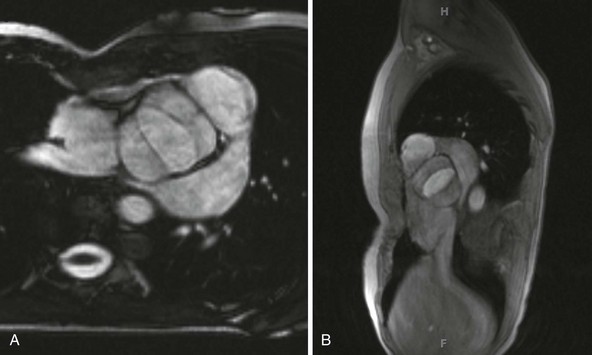
A, Short-axis view; B, sagittal view.
Disease Course and Outcomes
PATIENTS WITH BAV AND NO SIGNIFICANT AORTIC VALVE DYSFUNCTION (n = 212) *
PATIENTS WITH BAV WITH A SPECTRUM OF VALVE FUNCTION (n = 642) †
Mean follow-up, yrs (range)
15 ± 6 (0.4-25)
9 ± 5 (2-26)
Mean age at baseline, yrs
32 ± 20
35 ± 16
Outcomes:
Overall survival
90 ± 3% at 20 yrs
96 ± 1% at 10 yrs
Cardiac deaths
6.6%
3 ± 1%
Aortic valve or ascending aorta surgery
27 ± 4%
22 ± 2%
Cardiovascular medical events
33 ± 5%
Not available
Aortic dissection
0
2 ± 1%
Hospital admission for heart failure
7 ± 2%
2 ± 1%
Endocarditis
2%
2%
Predictors of outcomes
Predictors of cardiac events (medical and surgical)
Age ≥50 yrs
Valve degeneration
Age >30 yrs
Moderate or severe AS or AR
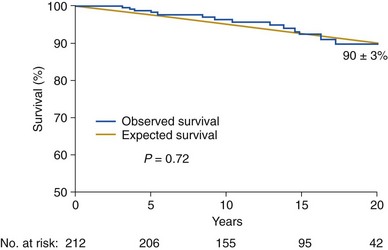
Blue line represents subjects with bicuspid aortic valve disease compared with an age- and sex-matched control population (yellow line). The numbers at the bottom indicate the patients at risk for each interval. The survival (+SE) is indicated 20 years after diagnosis. (From Michelena HI, Desjardins VA, Avierinos JF, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008;117:2776–84.)
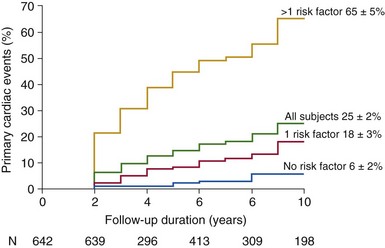
Risk factors included: age >30 years, moderate or severe aortic regurgitation, and moderate or severe aortic stenosis. (From Tzemos N, Therrien J, Yip J, et al. Outcomes in adults with bicuspid aortic valves. JAMA 2008;300:1317–25 [original content]; and Siu SA, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol 2010;55:2789–2800.)![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree