The temporal effect of heart failure (HF) hospitalization occurring at different time periods before implantation has not yet been studied in detail. The aim of the present study was to investigate the potential association between time from last HF hospitalization to device implantation and effects on subsequent outcomes and benefit from cardiac resynchronization therapy with a defibrillator (CRT-D). Multivariate Cox models were used to determine the temporal influence of previous HF hospitalization on the end point of HF or death within all left bundle branch block implantable cardioverter-defibrillator (ICD) and CRT-D patients enrolled in the Multicenter Automatic Defibrillator Implantation Trial–Cardiac Resynchronization Therapy (MADIT-CRT) trial (n = 1,250) and to evaluate the clinical benefit of CRT-D implantation, comparing CRT-D patients with ICD patients within each previous HF hospitalization group. The patients with previous HF hospitalization ≤12 months before device implantation had the greatest incidence of HF or death during 4-year follow-up (31%), while those with previous HF hospitalization >12 months and those with no previous HF hospitalization had similar lower rates of HF or death (22% and 24%, respectively). All patients treated with CRT-D derived significant clinical benefit compared with their ICD counterparts, regardless of time of previous hospitalization (hazard ratios 0.38 [no previous hospitalization], 0.49 (≤12 months), and 0.45 (>12 months); p for interaction = 0.67). In conclusion, in the present study of patients with mild HF with prolonged QRS intervals and LBBB, a previous HF hospitalization ≤12 months was associated with increased risk for HF or death compared with >12 months and no previous HF hospitalizations. The clinical benefit of CRT-D was evident in all patients regardless of time from last HF hospitalization to implantation compared with ICD only.
The benefit of cardiac resynchronization therapy with a defibrillator (CRT-D) has been substantiated in major randomized trials, with reductions in heart failure (HF) hospitalizations and mortality along with increased patient quality of life. Currently, there is much emphasis on appropriate patient selection for CRT-D implantation, as studies have shown that patients without left bundle branch block (LBBB) do not derive benefit from CRT-D and may even have worse outcomes. HF hospitalizations have been shown to be associated with worse outcomes and less reverse remodeling. However, the influence of HF hospitalizations occurring at different time periods before CRT-D implantation on outcomes and benefit of CRT-D has not been investigated. The present study was designed to investigate the association between time from last HF hospitalization to device implantation on subsequent outcome and CRT-D benefit in the Multicenter Automatic Defibrillator Implantation Trial–Cardiac Resynchronization Therapy (MADIT-CRT) population. We hypothesized that a recent HF hospitalization before CRT-D implantation would be associated with worse outcomes and reduced CRT-D benefit.
Methods
The design, protocol, and results of MADIT-CRT have previously been published. Briefly, MADIT-CRT was a randomized, multicenter trial designed to determine the possible benefit of implanting a CRT-D device compared with an implantable cardioverter-defibrillator (ICD) in patients with mild HF, prolonged QRS duration and depressed left ventricular ejection fractions. From December 22, 2004, through June 24, 2009, the trial enrolled 1,820 patients from 110 different centers in the United States, Canada, and various European countries. Patients were randomized in a 3:2 ratio for implantation with a CRT-D or an ICD device, respectively. Enrollment criteria dictated that patients had to have left ventricular ejection fractions ≤30%, have QRS durations ≥130 ms, and be in New York Heart Association (NYHA) class I or II with ischemic cardiomyopathy or NYHA class II with nonischemic cardiomyopathy. Patients were excluded from the trial if they had preexisting indications for device implantation, were in NYHA class III or IV, had undergone coronary artery bypass graft surgery or percutaneous coronary intervention, had enzyme-positive myocardial infarctions <3 months before enrollment, and had experienced atrial fibrillation <1 month before enrollment.
The present substudy of MADIT-CRT aimed to assess whether HF hospitalizations within different time periods before implantation affected the risk for HF or death and the benefit of CRT-D compared with an ICD. Numerous studies have substantiated that the effect of CRT-D is seen mainly in patients with LBBB QRS morphology. Therefore, the present study population was limited to patients with LBBB QRS morphology. Of the original 1,820 patients, 528 were excluded because of non-LBBB QRS morphology, and 42 were excluded because of lack of data on previous HF hospitalization. Therefore, the present study included 1,250 patients with LBBB, with 746 CRT-D patients (59.7%) and 504 ICD patients (40.3%).
Previous HF hospitalization was defined as last HF hospitalization before enrollment. Three distinct previous HF hospitalization groups were defined: no previous HF hospitalization, previous HF hospitalization ≤12 months before enrollment, and previous HF hospitalization >12 months before enrollment. To evaluate CRT-D benefit, CRT-D patients were compared with their ICD counterparts within each previous HF hospitalization group.
For the present study, we used the combined end point of nonfatal HF events or all-cause mortality (HF or death), whichever came first, as done in the primary study. Adjudication for the primary end point was conducted by an independent mortality and HF committee, as previously described.
Continuous variables are expressed as mean ± SD, and categorical variables are summarized as frequencies and percentages. Clinical baseline characteristics were compared among patients with LBBB treated with CRT-D or ICDs who had no previous hospitalization, previous HF hospitalization ≤12 months, and previous HF hospitalization >12 months. Kruskal-Wallis tests were used to evaluate significant differences between groups of continuous variables, and Fisher’s exact tests and chi-square tests were used for evaluating significant differences involving dichotomous variables.
Kaplan-Meier graphs were used to determine the cumulative probability of HF or death in CRT-D and ICD patients combined, within the 3 previous hospitalization groups. Multivariate Cox proportional hazards regression analyses were used to evaluate the temporal association between different previous hospitalization groups and the end point.
Subsequent analyses were conducted testing CRT-D benefit. Within each group of prior HF hospitalization, Kaplan-Meier graphs were used to compare CRT-D patients with their ICD counterparts, and subsequently multivariate Cox proportional hazards regression models were applied to evaluate the clinical benefit of CRT-D implantation, comparing CRT-D with ICD patients within the different previous hospitalization groups.
Adjustment variables within the models were determined on the basis of best subset regression analysis, which looked at potential confounding variables that affected the end point. The limit for entry into the model was set at p <0.05. Adjustment variables that affected the end point of HF or death included implantation of CRT-D or ICD, diabetes, glomerular filtration rate ≥60 ml/min/1.73 m 2 , left atrial volume index by body surface area, and QRS duration ≥150 ms. Adjusted hazard ratios, their 95% confidence intervals, and p values are reported, with a significance limit set at p <0.05.
Interactions were tested systemically for HF or death between baseline characteristics and previous HF hospitalization. Taking multiple comparisons into account, we set a significance limit for interactions at p <0.01.
Analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, North Carolina).
Results
Of 1,820 patients in the original MADIT-CRT trial, 1,250 (68.7%) had LBBB QRS morphology, with 746 patients treated with CRT-D and 504 with ICDs. The baseline characteristics of the 1,250 patients with LBBB subcategorized into no previous HF hospitalization (n = 774), previous HF hospitalization ≤12 months (n = 253), and previous HF hospitalization ≥12 months (n = 253) before CRT-D implantation are listed in Table 1 . In general, the group with previous HF hospitalization within 12 months before device therapy had more severe cardiac disease than the other 2 groups.
| Variable | Prior Heart Failure Hospitalization | ||
|---|---|---|---|
| No (n=774) | ≤12 Months (n=253) | >12 Months (n=223) | |
| CRT-D assigned treatment | 455(59%) | 151(60%) | 140(63%) |
| Women | 215(28%) | 82(32%) | 84(38%) * ¶ |
| Age (years) | 64.8±10.7 | 63.7±11.0 | 62.2±11.2 * ¶ |
| Heart rate (bpm) | 68.1±11.0 | 69.1±11.0 | 67.9±10.6 |
| Systolic blood pressure (mmHg) | 124.1±17.4 | 121.0±17.1§ | 119.8±15.8 * ¶ |
| GFR (mL/min/1.73 m 2 ) | 71.4±20.1 | 67.1±20.0 § | 65.9±21.1 * ¶ |
| Ischemic cardiomyopathy | 353(46%) | 120(47%) | 73(33%) * ¶ † |
| Diabetes mellitus | 223(29%) | 77(31%) | 72(32%) |
| Prior myocardial infarction | 249(33%) | 88(35%) | 58(26%) † |
| Prior atrial arrhythmias | 71(9%) | 39(16%) § | 28(13%) * |
| Prior ventricular arrhythmias | 41(5%) | 22(9%) § | 16(7%) |
| NYHA Class >II <3 months prior to enrollment | 40(5%) | 54(22%) § | 38(18%) * ¶ |
| QRS duration (ms) | 161.8±18.2 | 163.9±20.4 | 165.5±21.0 ¶ |
| Left ventricular ejection fraction | 23.9±5.3 | 22.9±5.4 § | 23.1±5.3 * ¶ |
| LVESV indexed by BSA (ml/m 2 ) | 88.7±23.7 | 91.6±25.3 | 94.9±26.2 * ¶ |
| LAV indexed by BSA (ml/m 2 ) | 46.5±10.0 | 47.1±10.6 | 49.0±10.0 * ¶ † |
| ACE-I or ARB | 741(96) | 244(96) | 217(97) |
| Aldosterone antagonist | 199(26%) | 114(45%) § | 108(48%) * ¶ |
| Anti-arrhythmic drugs | 39(5%) | 30(12%) § | 15(7%) * |
| Beta-blocker | 721(93%) | 238(94%) | 216(97%) ¶ |
| Digitalis | 185(24%) | 67(26%) | 97(43%) * ¶ † |
| Diuretic | 440(57%) | 221(87%) § | 185(83%) * ¶ |
HF or death events were documented in 268 of 1,250 patients with LBBB (21.4%) implanted with either CRT-D or ICD devices. During the course of the study, 114 patients (9.1%) died, with no difference among the 3 previous HF hospitalization groups (p >0.05).
The Kaplan-Meier cumulative probability of HF or death for the previous hospitalization groups is presented in Figure 1 . The patients with previous HF hospitalization ≤12 months before device implantation had the greatest risk for HF or death during 4-year follow-up, while those with previous HF hospitalization >12 months and those without HF hospitalization before device implantation exhibited similar lower risk for HF or death ( Figure 1 ).
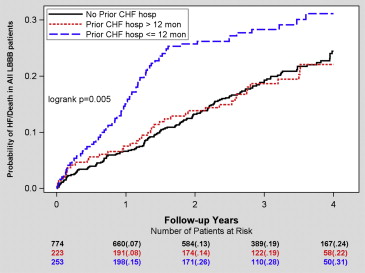
Multivariate Cox hazard regression analyses with adjustment for relevant covariates revealed that HF hospitalization ≤12 months before device implantation was associated with a significant 46% increased risk for HF or death compared with patients without previous HF hospitalization. Similar results were seen when comparing the risk for HF or death between previous HF hospitalization ≤12 months and previous HF hospitalization >12 months ( Table 2 ).
| Variable | Heart Failure/Death | |||
|---|---|---|---|---|
| # of Events/# of Patients | Hazard Ratio ∗ | 95% Confidence Interval | P-value | |
| ≤12 months vs. No prior HF hospitalization | 224/1027 | 1.47 | 1.10-1.95 | 0.009 |
| >12 months vs. ≤12 months | 115/476 | 1.50 | 1.02-2.19 | 0.039 |
| >12 months vs. No prior HF hospitalization | 197/997 | 0.98 | 0.70-1.38 | 0.909 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


