The first percutaneous vascular intervention was performed in the superficial femoral artery (SFA) by Charles Dotter in 1964 using a series of relatively crude dilatation devices. Despite the long-term success of this first intervention (the SFA remained open until the patient’s death from heart failure 3 years later) and numerous technological advances, endovascular revascularization of the infrainguinal vessels has been plagued by suboptimal long-term patency rates (1–4). Unfortunately, stenting in this location has not yielded the favorable results obtained in the iliac arteries or in the coronary circulation. A number of reasons for this failure have been proposed. First, peripheral artery disease (PAD) of the infrainguinal vessels tends to be diffuse, is often associated with severe calcification, and has a high propensity for progression to occlusive disease (5). Second, peripheral vessels are subject to a series of complex biophysical forces (6) that are dramatic and appear to impact the success of endovascular stenting and other revascularization strategies. Third, current stent platforms are not designed to withstand these complex forces resulting in stent fracture, which is thought to be associated with in-stent restenosis (7). Therefore, many operators continue to use angioplasty alone as the primary strategy for infrainguinal revascularization, a technique that is associated with high restenosis rates, particularly in the treatment of long lesions. These are the factors that have led to interest in debulking techniques for the treatment of infrainguinal disease (8).
In clinical practice, debulking techniques may be broadly divided into two types: excisional atherectomy (i.e., physical removal of plaque) or atheroablation (i.e., disintegration or fragmentation of the plaque without its physical removal). These techniques may be used in conjunction with angioplasty and/or stenting, or as a stand-alone strategy (8). This chapter will review the four currently available atherectomy/atheroablative devices that are approved for use in the peripheral arteries.
DEBULKING TECHNIQUES—CLINICAL INDICATIONS
In clinical practice, debulking techniques in the infrainguinal vessels are used to allow for the effective acute treatment of certain anatomic disease subsets that otherwise would be difficult or impossible to treat. As a method that can minimize plaque shifting, its application is well suited in ostial SFA disease to decrease the risk of plaque shift into the profunda femoral artery. Heavily calcified plaque is another important subset, where routine angioplasty and/or stenting is often associated with a suboptimal acute result. Debulking techniques typically allow a successful acute result without the need for stenting. Hence, their use at locations where stent use should be avoided, such as where joints are located (i.e., common femoral [CFA] and popliteal artery), is desirable. Finally, given that long overlapping stents appears to be a significant risk for the development of stent fractures and/or in-stent restenosis, the ability of debulking therapies to obviate stenting is of particular interest in patients with severe diffuse disease of the SFA/popliteal artery.
EXCISIONAL ATHERECTOMY
Directional Atherectomy-Silverhawk Atherectomy Catheter
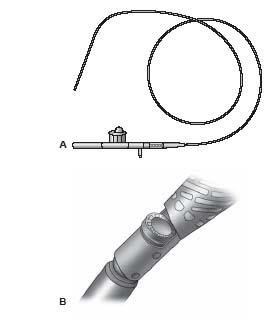
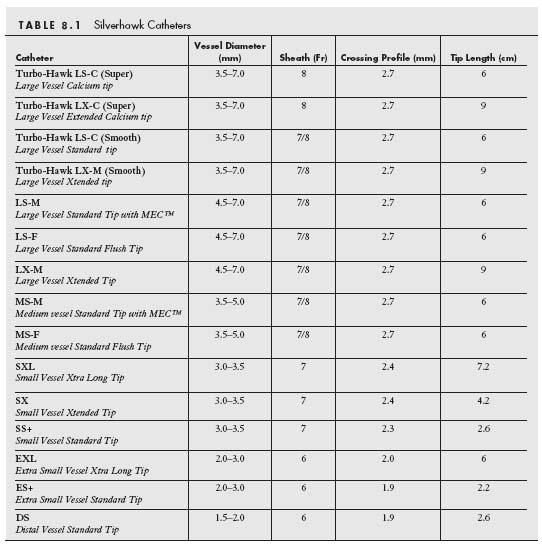
In June 2003, the FDA gave 510(k) approval to the Silverhawk Plaque Excision System (Fig. 8.1) (ev3, Plymouth, MN) for the treatment of PAD (9). This device uses a carbide cutter at a variable height (depending on catheter type) that rotates at a speed of 8000 rpm and engages the plaque via a hinge system, in contrast to earlier devices that used balloon apposition. There are currently 12 available models of the Silverhawk family of atherectomy catheters that allow for the treatment of diseased vessels ranging from 1.5 to 7.0 mm in diameter (Table 8.1) and for the treatment of lesions of various lengths (i.e., Standard nose-cone length for shorter lesions and eXtended nose-cone length for longer lesions). The nomenclature of these catheters can be confusing but can be explained as follows. The first letter refers to the vessel size in which the catheter is used (i.e., L, large; M, medium; S, small; E, extra small). The second letter refers to the length of the nose cone (S, standard length; X, extended length). The third letter generally refers to the variations in the design of the nose cone (F, standard nose-cone design; M, presence of MEC technology in which laser-drilled micro vent holes in the nosecone maximize tissue storage in the nose cone; C, special modification of the cutter and nose cone to allow for treatment of calcified lesions).
The device consists of a monorail low-profile catheter (advanced over any 0.014″ wire, preferably an extra-support wire) and a palm-sized drive unit with an on/off switch that activates and deactivates the cutter, respectively, and an additional switch to raise the carbide cutter from the nosecone such that atherectomy can be performed. (Fig. 8.1). Atheroma is cut and collected in a hinged nose cone that is located distal to the cutter. The number and length of cuts is determined by the operator and the device can be rotated to allow circumferential cutting of the plaque.
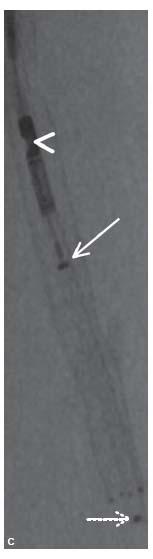
Figure 8.1 • Silverhawk Plaque Excision System. A: The TurboHawk atherectomy catheter that uses a carbide cutting blade specifically designed for treating calcium. B: Magnified view of the carbide cutting blade (arrowhead). C: Angiographic image of the TurboHawk atherectomy device with its cutting blade (arrowhead) and the plunger (arrow) used to pack the atheroma into the nose cone of the device. The tip of the nosecone is indicated by interrupted white arrow.
PRACTICAL USE
The recommended sheath size for each of the Silverhawk catheters is summarized in Table 8.1 (i.e., 6 to 8 Fr.). Although an 8 Fr. sheath is recommended for the larger catheters (i.e., LS and LX), the authors have generally used 7 Fr. sheaths to deliver these catheters. As a general rule, the large catheter group (i.e., LS-C, LX-C, LS-M, and LX-M) is used in the CFA, SFA, and larger caliber popliteal arteries. The medium catheter (MS-M, MS-F) is used in the popliteal artery and the small catheter group (SS+, SX, and SXL) is suitable for use in the proximal segment of good caliber tibial vessels. The extra-small catheters (ES+, EXL, and DS) are suitable for use in the mid and distal tibial vessels. Once the lesion has been crossed, a 0.014″ wire (preferably an extra-support wire, e.g., Grand Slam, Abbott Vascular, Santa Clara, CA) is required to allow for subsequent delivery of the catheter. Excisional atherectomy inherently has a risk of distal embolization, and therefore a careful assessment of the runoff should be made at baseline. In cases in which the use of an embolic protection device is thought necessary, the authors use a Spider FX® (eV3, Plymouth, MN) or Emboshield (Abbott Vascular, Santa Clara, CA) filter. The filter should be at least 1 mm larger than the distal vessel in which it is placed. The Spider FX filter permits crossing of the lesion with any 0.014″ wire followed by exchange for the Spider filter and wire combination. The latter wire provides reasonable support for subsequent catheter delivery. Of note, if a 0.035″ wire is used to cross the lesion, a 4 Fr. glide catheter or 0.035″ Quickcross catheter can be used to exchange the 0.035″ wire for the Spider FX filter, a technique which can shorten the procedural time. When choosing the location of the landing zone of the filter of choice, it is important to be cognizant of the length of the nosecone of the Silverhawk catheter being used. It is necessary for the filter to be placed at least 1 to 2 cm plus the length of the nose cone inferior to the distal margin of the lesion being treated. For example, for mid and distal popliteal lesions, the filter will need to be placed in a tibial vessel.
Prior to introduction into the body, the device should be flushed with heparinized saline and the drive unit should be attached to the catheter and activated to ensure proper functioning of the cutter. The catheter is then advanced such that the cutter is located approximately 1 cm superior to the proximal margin of the lesion. In this location, the tip and body of the nose cone will be situated across the proximal portion of the target lesion. Rarely, low-pressure angioplasty (using 2.0 to 3.0 mm balloon) may be required prior to atherectomy to permit device advancement, particularly when treating long occlusions or heavily calcified lesions. The authors use the roadmap function in combination with radio-opaque marker tape (e.g., LeMaitre® tape, LeMaitre, Burlington, MA) to help delineate the target area for treatment. The directionality of the cutter is adjusted as desired by rotating the back end of the catheter. When using a 2D angiographic image, one can only reliably assess whether the cutter is broadly directed medially, laterally, anteriorly, or posteriorly. The cutter is then activated and advanced in a smooth manner and at a constant speed (1 to 2 mm/sec) across the lesion. The slower the speed of catheter advancement, the deeper the cut, and vice versa. In general, the authors stop after no more than 5 cm of lesion treatment to pack the nosecone of the device. This likely reduces the risk of distal embolization of plaque elements. Following a single cut in a given direction, the deactivated device is brought back to the starting point. The device should never be pulled back while activated. The direction of the cutter can then be changed and the process repeated. Following a series of cuts, the nosecone will become full of plaque. In the larger caliber devices, this can be assessed on x-ray by noting the position of the radio-opaque marker on the plunger in the nosecone (Fig. 8.1C). The closer this marker is to the cutter, the more full the nosecone. The device is removed and the plaque removed from the nosecone using a variety of techniques, depending on the catheter type. The device is then reinserted and additional cuts can be made as desired.
CLINICAL DATA
The Treating Peripher Als with Silverhawk: Outcomes CollectioN (TALON) is the largest prospective registry of Silverhawk use involving 601 patients from 19 different U.S. centers (10,11). All data was entered voluntarily, and outcomes were operator-reported without independent core-laboratory adjudication. Treatment was confined to the infrainguinal region (i.e., above- and below-knee). The dominant indication for treatment was claudication, with approximately one third of patients having critical limb ischemia (CLI). Short and midterm outcomes of this registry have been reported. Acute procedural success was achieved in nearly 98% of cases. Target lesion revascularization (TLR) occurred in 10% and 20% of patients at 6- and 12-month follow-up, respectively.
In a separate analysis from this same registry including only patients with CLI, procedural success was achieved in 99% of cases (12). The primary endpoint of death, myocardial infarction, unplanned amputation, or repeat target vessel revascularization (TVR) occurred in 1% of the 69 patients at 30 days and 23% at 6 months. Notably there were no unplanned amputations in this high-risk group and amputation was avoided in 92% of limbs at 30 days and 82% of limbs at 6 months.
PROS
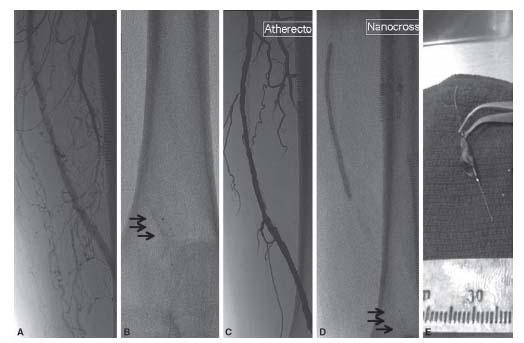
Given the directionality of this device and the operator controlled cuts, this device functions well in the treatment of eccentric disease (Fig. 8.2) and in locations where plaque shift may be poorly tolerated (i.e., SFA ostium). Because of the luminal gain that can be achieved with the larger catheters, it is also particularly useful in the treatment of CFA disease. In terms of treating heavily calcified disease, the authors experience is that the TurboHawk catheters are very effective (Fig. 8.3) and achieve results superior to that achieved using either Laser or the Pathway Jetstream catheter and rival those achieved with the Cardiovascular Systems Inc. (CSI) diamondback catheter. No console is required for this catheter, which eliminates the initial funding issues associated with other debulking technologies.
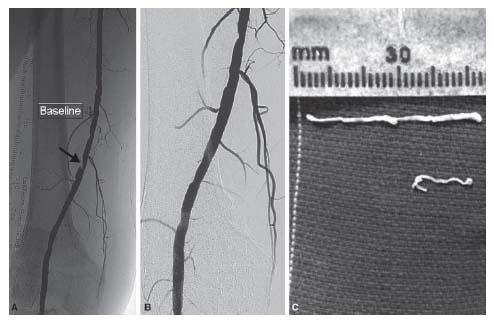
Figure 8.2 • A: Focal eccentric lesion of the superficial femoral artery at Hunter’s Canal (arrow). B: Excellent angiographic result post atherectomy alone using a TurboHawk® catheter. C: Atheromatous debris collected from the TurboHawk device.
Figure 8.3 • A: Severe calcific disease of the distal portion of the superficial femoral artery. B: After the disease was crossed using a 0.014″ wire, a Spider FX filter (black arrows) was deployed distal to the lesion. C: Angiogram showing significant luminal gain post TurboHawk atherectomy. D: Given the extensive disease, a 4.0-mm angioplasty balloon was inflated at 3 atm for 5 min following atherectomy with little change in angiographic appearance (not shown). E: Image of the significant calcified atheromatous debris captured by the filter in this case. Runoff angiography disclosed no evidence of distal embolization.
CONS
The spectrum of catheters required to treat the range of vessel diameters and lesion types is problematic, as these catheters are typically purchased and laboratory costs are significant (~$3000 to $3500/catheter). This device requires removal from the body in order to clean the nosecone of atherectomized material; therefore, the treatment of long segments of disease is associated with prolonged procedural times and the risk of trauma to the catheters. This trauma can be associated with catheter failure and the need to use a new catheter, which adds significantly to the cost of the procedure. The directionality of the cutter probably does increase the risk of trauma to the media with this device. Acute perforations and arterio-venous fistula formation, and late pseudoaneurysm formation and perforation have been reported (see chapter 16), and reflect the real potential for trauma to the media using this device. These potential complications suggest that use of this device in the subintimal space should be carried out with caution. As with other atherectomy devices, distal embolization has also been reported (10,13).
Jetstream Peripheral Vascular Atherectomy System
First approved by the FDA in 2008, the Jetstream Peripheral Vascular Atherectomy System® (Pathway Medical Technologies, Kirkland, WA) (Fig. 8.4) is unique in that it currently is approved for atherectomy in infrainguinal vessels and for thrombectomy of upper and lower extremity vessels.
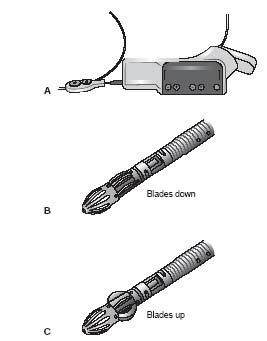
The atherectomy function of the catheter is achieved by the presence of five stainless steel blades at the catheter tip that rotate at a speed of 70,000 revolutions per second. These blades are unique in that they are expandable (i.e., they assume a tangential position (blades down) when the catheter rotates in a clockwise direction with a nominal diameter of 2.1 mm and assume a radial position (blades up) when the catheter rotates in a counterclockwise direction with a maximum diameter of 3.0 mm). The blades are specifically designed to achieve differential cutting of plaque elements from the normal elastic vessel wall. The catheter also has an aspiration function that is achieved by separate pumps located in a console mounted on an IV pole, which infuse saline through the catheter and aspirate debris from the catheter tip. One of the major modifications between the first and second (G2) generations of this device was in the location of the aspiration ports. By moving these ports from the distal tip of the catheter to just proximal to the blades resulted in improved aspiration and resolution of the issue of “vessel suckdown” in which advancement of the activated catheter appeared to be hampered by excessive suction of the vessel wall by the catheter tip. The dual function of this catheter in performing both atherectomy and aspiration explains its approval for the treatment of both atheroma and thrombus.
Figure 8.4 • Jetstream Peripheral Vascular Atherectomy System. A: The Jetstream atherectomy system showing the handheld control. B: The catheter tip in the blades down position. C: The catheter tip in the blades up position.
PRACTICAL USE
There are currently two Jetstream catheters available. The G3 catheter, which is intended for use in the CFA, SFA and popliteal vessels, can be used in the “blades down” position (Fig. 8.4B) in vessels as small as 3.0 mm and in the “blades up” position (Fig. 8.4C) in vessels with a reference vessel diameter of 4 mm. The recently released G3 SF catheter was designed for use in the below-knee vessels, with a reference vessel diameter of 2.0 to 3.0 mm. Both catheters are delivered using an 0.014″ wire in an over-the-wire (OTW) system, and require 7 Fr. sheath for delivery (Table 8.2). A dedicated 0.014″ wire (Pathway Jetwire™) was recently released by the manufacturer of the Pathway atherectomy system. Where this wire is not available, specific wires that are compatible with the catheter are recommended by the manufacturer (Table 8.2). From this latter list of wires, in the authors’ experience, the Viper family of wires (CSI, St Paul, MN) interact best with this catheter. Of note, the use of this device in the subintimal space is not recommended by the manufacturer, but has been performed (Fig. 8.5). However, if a re-entry device is used to regain entry to the true lumen, the authors would not recommend the use of this device. The device has been tested with the Spider FX embolic protection device (ev3) and the Spider wire is compatible with Jetstream G3 catheter. However, other embolic protection devices such as the Emboshield Nav6 (Abbott Vascular) have also worked well with this device.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree