Technique of Extrapleural Pneumonectomy for Diffuse Malignant Pleural Mesothelioma
Lambros Zellos
David J. Sugarbaker
Extrapleural pneumonectomy (EPP) was described initially for the treatment of tuberculous empyema in the 1940s by Sarot.19 Since that time indications have expanded beyond benign conditions into malignant diseases such as diffuse malignant pleural mesothelioma (DMPM), pleural sarcoma, thymoma with pleural implants, and even non-small-cell lung cancer with pleural involvement. While the indications of the surgery remain controversial in these rare diseases, the technique has undergone refinement since Butchart’s report in 1976,3 which published a mortality rate of 30%. More recent series by DeLaria,6 DaValle,5 Ruffie,15 Sugarbaker,26 Allen,1 and Rusch18 have consistently shown a much improved mortality rate with most series reporting rates below 10% and some below 5%.
In this chapter we describe our approach to patient selection, preoperative preparation, surgical resection and reconstruction, and postoperative management of complications after EPP in patients with DMPM. The bulk of our experience with EPP does not involve neoadjuvant therapy. As neoadjuvant therapy is becoming more popular in the treatment of mesothelioma, further reports are needed to address its impact on morbidity and mortality after EPP.
Patient Selection
Appropriate patients for EPP are under 75 years of age and have good functional status with a Karnofsky score >70. We have made exceptions for some patients over age 75 if they are active and have excellent cardiopulmonary status. Liver and renal function should be normal. The echocardiogram should demonstrate good right and left ventricular function with an ejection fraction >45% and absence of pulmonary hypertension. If pulmonary hypertension is suggested, right heart catheterization should be performed. In borderline cases, cardiopulmonary exercise testing may be needed to determine risk of mortality. Predicted postoperative forced expiratory volume in 1 second (FEV1) should be >0.8 L. Patients who have preoperative FEV1 <2.0 L undergo a quantitative ventilation/perfusion scan to calculate the value for postoperative pulmonary function. Cardiac stress tests are obtained as indicated by the patient’s risk factors for postoperative cardiac events. Deep venous thrombosis is ruled out preoperatively with vascular ultrasound.
The diagnosis of DMPM is confirmed at our institution with review of the pathology slides. Patients whose diagnosis is based on cytology alone undergo multiple pleural biopsies via thoracoscopy. We have had several patients who were diagnosed with mesothelioma based on cytologic examination alone, only to be correctly diagnosed later with one of various adenocarcinomas (i.e., lung, ovarian, etc.) after open pleural biopsy.
Resectability is determined by assessing chest wall, mediastinal, vascular, and diaphragmatic invasion using magnetic resonance imaging (MRI). While patients referred for thoracic consult will already have undergone computed tomography (CT) of the chest on presentation, Patz14 has shown that MRI provides added value to the CT scan. Positron emission tomography (PET)-CT scan is also obtained to rule out unsuspected distant disease, as reported by Flores.7 Despite all of these tests, we have had false-positive and false-negative readings of chest wall, great vessel, or aortic invasion as well as transdiaphragmatic extent. Consequently we have adopted a rather liberal policy of exploration via thoracotomy and sometimes laparoscopy to assess resectability, an approach that is shared by other groups (Rusch and Venkatraman17). Applying this liberal policy of exploration, our resectability rate historically has been 80%.
Right Extrapleural Pneumonectomy
Intraoperative monitoring involves pulse oximetry, electrocardiographic (ECG) monitoring, arterial lines, and central venous lines. A thoracic epidural is routinely used as well. A double-lumen tube is used for selective ventilation. The nasogastric tube assists in the identification of the esophagus intraoperatively and is left in place postoperatively. The patient is placed in the full lateral position.
If transdiaphragmatic extension needs to be ruled out two options exist: (a) laparoscopy or (b) limited subcostal incision in line with the future thoracotomy incision. When frank
peritoneal involvement is ruled out, the thoracotomy can be performed.
peritoneal involvement is ruled out, the thoracotomy can be performed.
The extrapleural pneumonectomy is conducted via a single skin incision and single thoracotomy. The steps are as follows: (a) incision and exposure of the parietal pleura, (b) dissection in the extrapleural plane to separate the tumor from the chest wall and mediastinum, (c) dissection of the diaphragm off the chest wall and peritoneum, (d) division of the anterior and inferior pericardium, (e) division of the pulmonary artery and veins, (f) division of the posterior pericardium, (g) dissection and division of the bronchus, (h) mediastinal lymph node dissection, (i) reconstruction of the diaphragm and pericardium, and (j) closure.
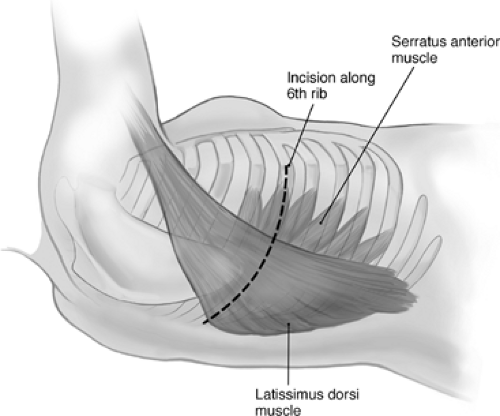
The thoracotomy incision is made along the sixth rib, with division of the lattissimus and serratus muscles and resection of the sixth rib (Fig. 69-1). The extrapleural dissection begins over the bed of the resected rib and is advanced toward the apex. Most of the dissection is done bluntly with sharp dissection using scissors or cautery as needed. Dissected areas are packed with pads to limit blood loss. By dissecting the lateral, anterior, and posterior parietal pleura toward the apex, the tumor is gradually mobilized fully until the apex is reached. The tumor is then dissected off the apex and subclavian vessels as needed. Structures that may need to be protected from injury include the mammary vessels (Fig. 69-2) and the subclavian vessels. As the dissection continues inferiorly and medially, the superior vena cava, azygos vein, airway, and esophagus also need to be protected (Fig. 69-3).
The dissection is continued inferiorly toward the diaphragm. The best location to start the diaphragmatic dissection is at the anterior costophrenic angle. The muscle fibers are bluntly detached circumferentially from the chest wall, and the peritoneum is identified (Fig. 69-4).
The dissection is then continued toward the medial attachment of the diaphragm to the pericardium. The pericardium is entered near the anterior costophrenic angle, and the pericardial incision is extended superiorly toward the superior vena cava and posteriorly toward the inferior vena cava (Fig. 69-5). The pulmonary vessels are then divided intrapericardially using the endovascular staplers (Fig. 69-6).
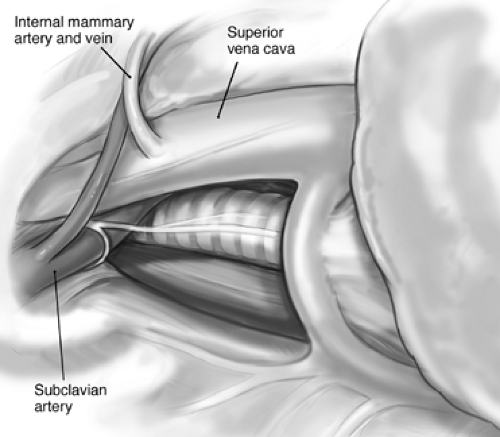 Figure 69-2. Structures to avoid during pleural dissection of tumor include the internal mammary artery and vein, which originate from the subclavian artery and superior vena cava, respectively. |
The posterior diaphragmatic attachments are divided, leaving a rim of the crus on the esophagus, and the diaphragmatic resection is complete. The pericardial resection is completed by dividing the pericardium posterior to the pulmonary vessels toward the carina. The subcarinal nodes are dissected and the right mainstem bronchus is prepared for division. We use staplers under direct bronchoscopic examination to divide the airway (Fig. 69-7).
The specimen is removed and the chest is inspected for bleeding. We find the argon beam coagulator (Valleylab, Boulder, CO) to be very helpful to stem bleeding on the raw chest wall surface. In addition, local hemostatic agents such as fibrin glue are applied throughout the chest cavity. The mediastinal lymph node dissection is completed. The chest is inspected for any
gross residual disease. Tumor nodules that cannot be resected are delineated with clips to target the delivery of postoperative radiotherapy.
gross residual disease. Tumor nodules that cannot be resected are delineated with clips to target the delivery of postoperative radiotherapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree