Chapter 88
Technique
Endovascular Diagnostic
Eric J. Turney, Sean P. Lyden
Based on a chapter in the seventh edition by Kim J. Hodgson and Douglas B. Hood
Endovascular therapy has become an integral part of vascular surgery practice. The first part of any intervention is the diagnostic imaging. The choices made during diagnostic imaging can affect any subsequent interventions both positively and negatively. Planning for a diagnostic procedure should consider the future surgical and endovascular therapies that may be needed. For endovascular treatment, the diagnostic access artery must be large enough to accommodate the sheath size needed for the treatment devices and be sufficiently near the intended target. From the surgical standpoint, access sites should be chosen so as not to compromise future surgical targets should hematoma, dissection, or thrombosis occur.
Access
Choice of Site
Several general principles are important to guide access site choices. In general, the best arterial access sites should have a bone or bone prominence beneath the artery against which pressure can be applied on completion. When a bone or bone prominence is not present, external pressure may not occlude the arteriotomy because the artery is not fixed in its location. Entry sites should avoid diseased areas of an artery, especially those with significant calcification. In addition, access should be made on the top of the blood vessel away from side branches, bifurcations, or crossing veins.
In choosing an access site, the decision should be influenced by the size of the artery; the size of the desired sheath; the body location to be imaged; the stenoses or occlusion that may need to be crossed or avoided; and the length of diagnostic catheters, sheaths, balloons, wires, and interventional devices intended to be used. It is important to envision the entire case before starting to try to make sure that the operator has the devices needed to complete the case.
Ultrasound Guidance
No one method to correctly identify and access the blood vessel at the intended location has proved infallible. In obese patients, use of external skin markings to identify potential access sites is likely to lead to entry in an incorrect location. Ultrasound-guided vessel access has the ability to identify the intended vessel, to confirm patency, and to identify side branches.1 Once the proper location for puncture has been identified, local anesthetic is instilled and the entry needle introduced at an angle of 30 to 45 degrees.
Access Options
There are multiple choices to gain arterial access for arteriography. The common femoral artery (CFA) is the most commonly used site. Alternative sites include the axillary artery, which was frequently used in the past but has been replaced more recently with brachial and radial artery access as devices have become longer and smaller in profile. Alternative access for infrageniculate interventions can be obtained distally through the popliteal arteries or tibial arteries. Direct carotid and subclavian artery access has been used to deliver devices when other access sites are not available, but these two sites are associated with a higher risk of hemorrhage because of the inability to adequately compress the access site when the catheters or sheaths are removed.
Common Femoral Artery
The CFA is one of the most frequently used access sites for diagnostic angiography. Either an antegrade or retrograde approach can be undertaken, depending on the specific requirements of the procedure, anatomic characteristics, and body habitus. Retrograde femoral access is a reliable, safe option for a variety of diagnostic purposes because of its relatively large diameter, ease of compression, and options for image-guided puncture. With the advent of suture-mediated closure devices, the CFA has been used to successfully deliver devices as large as 26F. Antegrade access of the CFA has proved useful for infrageniculate interventions because of the straight-line approach, which increases pushability and torquability of catheters and wires.
Localization
There are several methods to identify the location of the CFA. Identifying an arterial pulse in the inguinal skinfold, without any adjunctive imaging, is the least reliable method and should be avoided except in thin patients with clear anatomy. In the obese patient, the inguinal fold is commonly much lower and overlies the superficial femoral artery (SFA). The inguinal ligament marks the beginning of the femoral artery and can be reliably identified in patients by use of bone landmarks. The inguinal ligament attaches to the anterior superior iliac spine and to the pubic tubercle. The femoral artery begins at the inguinal ligament and lies underneath it in the medial third (Fig. 88-1). The pulse palpable just under the inguinal ligament should be the CFA.
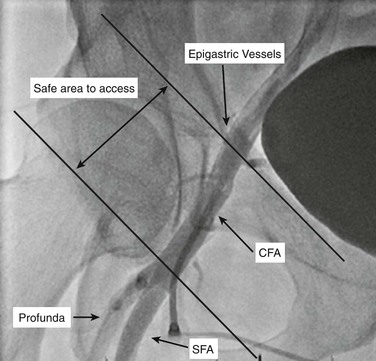
Figure 88-1 Femoral artery localization. Right anterior oblique angiogram demonstrates the correct zone of entry of the common femoral artery. The access should be below the epigastric branches and above the profunda femoris artery, between the oblique lines. CFA, Common femoral artery; Profunda, profunda femoris artery; SFA, superficial femoral artery.
Fluoroscopy.
Adding spot fluoroscopy of the femoral head to a technique of vessel palpation increases the safety and accuracy of CFA puncture.2 Depending on body habitus, the skin insertion site will be 1 to 4 cm caudal to the anticipated entry site in the CFA. The femoral artery crosses over the medial third of the femoral head (Fig. 88-2). One important caveat is the risk of parallax error when fluoroscopy is used (Fig. 88-3). Parallax error is greatest when the area of imaging is not centered and is a far distance away from the image detector.
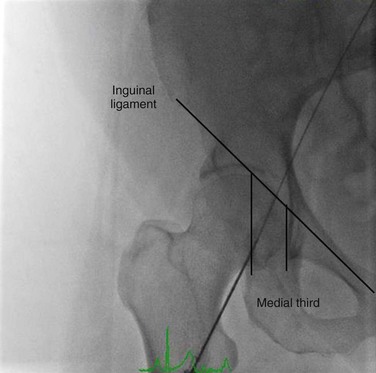
Figure 88-2 The common femoral artery lies over top of the medial third of the femoral head. A wire is in the femoral artery, noting its location relative to the femoral head below.
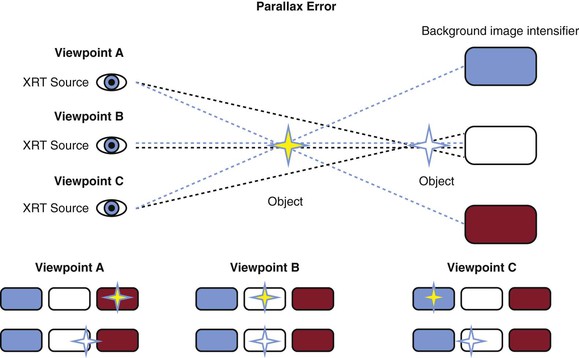
Figure 88-3 Parallax error is the change in the apparent position of an object when the position of the observer changes. The closer the object is to the background (the image intensifier), the degree of change in apparent position is lessened, as shown by the white star versus the yellow star.
If preoperative imaging, such as a computed tomographic angiography scan, has been obtained of the area of access, it may be possible to correlate surgical titanium clips or calcification with the fluoroscopic image to guide needle entry. The depth between the skin surface and the artery must also be considered. A longer distance from the skin to the vessel makes the angle of needle entry important to avoid an inadvertent high puncture above the inguinal ligament.
Ultrasound Guidance.
Ultrasound-guided direct visualization of the entry site is favored by many operators as the most accurate way to access all of the vasculature. With use of ultrasound, the CFA can be identified by localizing the inferior epigastric artery proximally and the SFA and profunda femoris artery (PFA) bifurcation distally. After patency of the vessel is confirmed, an area without significant plaque burden can be chosen. With the probe at a right angle to the skin, the depth from the skin to the artery should be measured. Knowing the depth of the artery from the skin allows the operator to select the distance to enter the skin away from the probe and the angle of entry for the needle to ensure that it punctures the top of the CFA in the area visualized. A probe guide can be used to help achieve proper angle and depth of the needle until skill is gained in performing this task. A common error of the novice is not to enter the artery at the site of visualization. This occurs when the needle enters either cranial or caudal to the area imaged and direct needle entry into the vessel is not visualized. Turning the probe parallel to the needle course or using curvilinear probes can effectively display the entire course of the needle tip from skin to within artery lumen.
Antegrade Access
Antegrade CFA access can be a valuable technique in patients with aortic bifurcations that are prohibitive to a contralateral retrograde CFA access and for tibial artery interventions, especially for crossing chronic total occlusions. Antegrade CFA puncture can be very difficult in obese patients because of body contour challenges. In obese individuals, upward retraction of the abdominal pannus either with the hand of an assistant or by tape placed in a cranial direction can improve chances for a successful access. It is also important to take into account the increased distance traveled through the subcutaneous adipose tissue, often necessitating puncture of the skin with a longer access needle well above the inguinal ligament. Needle entry close to the SFA origin leaves little working distance to attempt to direct the entry wire into the desired location. The wire tends to aim toward the posteriorly oriented PFA orifice because of the acute angle of entry. Success can be improved by puncture as close as possible to the origin of the CFA but still staying below the inguinal ligament. Micropuncture sets can allow placement of the inner portion of the transitional dilator to permit gentle pullback and safe use of a hydrophilic wire to be directed into the SFA.
Complications
Complications are more likely when access into the CFA is not in the correct place. Entry above the inguinal ligament in the external iliac artery increases the risk of access site bleeding, retroperitoneal hematoma, and pseudoaneurysm formation because of inadequate compression after the procedure. Closure devices are not designed to work with access through the inguinal ligament and are more likely to fail. Punctures of the distal CFA or beyond the bifurcation of the SFA and PFA can also lead to increased access site complications of hemorrhage, arteriovenous fistula, pseudoaneurysm, and vessel occlusion.
Popliteal Artery
Popliteal artery access has grown in popularity in the last decade as an alternative to cross SFA occlusions from a retrograde approach. The easiest way to access the popliteal artery is in the prone position directly behind the patella with ultrasound guidance. It is important to remember that the popliteal vein is commonly duplicated in this position to avoid errors in identification. In conjunction with a second access site with the patient in a supine position, the popliteal artery can be accessed by flexing the knee and externally rotating the hip. Regardless of patient positioning, either fluoroscopic or ultrasound guidance is required to accurately identify and to puncture the above-knee popliteal artery in the non-diseased location. Both fluoroscopy fade and roadmap modalities of current angiographic machines can be helpful to guide fluoroscopic access. Rotation of the image intensifier 90 degrees opposite of the original image can guide depth of the needle to improve accuracy of the puncture attempt. Unlike in other access areas, localization of a stable bone prominence against which to hold pressure during sheath removal is more difficult. Open space exists (which may not allow adequate compression) between the end of the femur and the tibial plateau and between the femoral condyles. Minimizing the access diameter can help lower bleeding risks when the access is removed. Other options for obtaining hemostasis on removal of the access include inflation of a nonocclusive external blood pressure cuff and internal balloon tamponade delivered through an alternative access.
Tibial Artery
Infrageniculate arterial access is not often used for purely diagnostic purposes; more often, it is part of an attempt to recanalize superficial femoral, popliteal, and tibial total occlusions.3 Fluoroscopic or ultrasound guidance can be used to identify the tibial arteries. Micropuncture access is recommended for tibial arteries to lower the risk of access vessel injury. Use of the transitional dilators, crossing catheters, or 3F sheaths can keep stable access and access diameter at a minimum. The posterior tibial artery is typically identified and accessed just above the medial malleolus. The posterior tibial artery is the least constrained in an arterial sheath and tends to be the most mobile, making it the most difficult tibial artery to enter. The dorsalis pedis artery lies lateral to the extensor hallucis longus muscle. The peroneal artery lies posterior to the sheath between the tibia and fibula and is fixed by the adjacent tissue. A great way to keep access site diameter to a minimum is to use a secondary proximal larger vessel access. Once an occlusion is crossed from a lower access, the wire can be captured from above with an angled catheter or snare, creating through-and-through access. Catheters and devices can then be delivered from the proximal larger access. The tibial access then can be removed and pressure held over the access site. A nonocclusive externally applied blood pressure cuff or internal balloon delivered from a superior access once again is an effective way to obtain hemostasis.
Axillary Artery
The axillary artery requires specific arm positioning to facilitate safe entry. The arm is elevated above the shoulder and flexed toward the head to rotate the humeral head to a position below the artery. Access is performed above the bone as the artery is palpated in the intramuscular groove between the coracobrachial and triceps muscles. The proximity to the brachial plexus is important as access site axillary sheath hematoma can lead to significant nerve compression and symptoms.4 This access is much less commonly employed than more distal upper extremity access.
Brachial Artery
Brachial artery access is performed with the arm supinated over the olecranon process. Entry into the artery should be over the olecranon process at the level of the antecubital crease on the medial side of the forearm overlying the lateral border of the brachial muscle. Ultrasonography can help define arterial size and anatomic variants before the vessel is punctured. A small percentage of patients will have a high takeoff of the radial artery off the brachial artery in the upper arm, leading to two pulses in the antecubital crease. Access of a small brachial or radial artery may lead to higher complication rates. Most individuals will accommodate up to 6F diameter sheaths in the brachial artery without difficulty, but larger diameter access is likely to lead to vessel occlusion and to require surgical repair of the entry point.5 Median nerve compression or injury is a risk of this access in 0.2% to 1.4% of cases.6
Radial Artery
The radial artery is now commonly used for coronary angiography. It can also be employed for hemodialysis access fistulography and visceral angiography. This access site has the advantage of easy hemostasis with minimal risk of bleeding. Typically, sheaths up to 6F can be advanced through the radial artery. Some authors have found instillation of vasodilators helpful in avoiding spasm of the vessel to aid in sheath or guide advancement.
Venous
Whereas venography for purely diagnostic purposes is less frequently performed, the techniques remain important as part of vena cava interruption, venous compression syndrome diagnosis, and venous thrombosis treatment. Access technique is similar to arterial in that venous anatomy runs in parallel to the arteries, but veins are commonly duplicated in the extremities. For this reason, ultrasound-guided puncture is even more helpful. Use of a syringe for aspiration to confirm venous entry is helpful; because of the lower pressure in the venous system, blood is not pushed out of the needle hub in many circumstances. A few anatomic locations deserve special mention.
The common femoral vein is the most commonly accessed location. It lies medial to the CFA, approximately 2 cm lateral to the pubic tubercle. The jugular vein is also commonly used and lies lateral to the carotid artery. The jugular vein can be accessed in between the sternal and clavicular heads of the sternocleidomastoid muscle lateral to the carotid pulse. Ultrasound-guided access will confirm vessel patency and absence of partial thrombosis and help ensure accurate needle placement, avoiding the adjacent artery. Ultrasound localization has proved much safer than anatomic localization in avoiding inadvertent arterial access and is being increasingly mandated in many hospitals as a quality initiative.
The popliteal vein is most often accessed in individuals with iliofemoral and femoropopliteal acute thrombosis as a prelude to initiation of therapy. The patient is placed in the prone position and the popliteal vein identified by ultrasound, often lying superficial and lateral to the artery. In the presence of thrombus, there often will not be blood return. In accessing a vessel with acute thrombus, the 0.018-inch wire should pass with minimal resistance, allowing placement of the transitional dilator.
Upper extremity venography is often performed to evaluate anatomy before dialysis access surgery as well as to evaluate anatomy and to treat central venous problems. Superficial veins may be directly cannulated. The basilic vein is a valuable access site because of its superficial course distal in the upper arm and relatively safe remote location from the artery.
Equipment
Access Needles
Arterial and venous access can be done with a single-wall or double-wall puncture needle technique. Single-wall puncture needles are available in either 18- or 21-gauge sizes. The 18-gauge needle will accept a 0.035-inch guide wire, whereas the 21-gauge needle will accept a 0.018-inch guide wire. The 21-gauge versions are commonly referred to as micropuncture needles. Most sheaths are introduced over a 0.035-inch wire, so when a micropuncture needle is used, a transitional dilator, which contains an inner dilator and a 4F or 5F introducer, is needed. When the 0.018-inch guide wire and inner dilator are removed, the remaining 4F or 5F introducer accepts up to a 0.035-inch or 0.038-inch wire, respectively, allowing placement of a traditional sheath. Micropuncture needles can make access to smaller vessels possible and can allow confirmation of appropriate access to the anterior surface of the correct and intended vessel before exchange to a larger bore device, such as a sheath. This has the potential to lower the risk of access-related vessel injury and access-related complications.
Double-wall puncture needles are two-component systems containing a blunt-tipped hollow needle with a beveled solid stylet that projects out from the end of the needle. The double-wall technique involves passing the needle through both the anterior and posterior vessel wall until contact is made with the underlying bone, followed by removal of the stylet. The beveled stylet is removed, and the hollow needle is withdrawn slowly until blood return is noted. Double-wall puncture needles are not widely used because of the unnecessary posterior wall vessel puncture.
Guide Wires
Insertion
Once access is achieved with a 0.035-inch needle, a floppy-tip or J-tip wire is typically used to enter the vessel. The floppy and J tips tend to permit atraumatic passage through the vessel until a stiffer portion of the wire enters to allow passage of a sheath. Fluoroscopic visualization of wire traversal is recommended to confirm its course in the intended vessel and that a side branch has not been entered or a dissection created. Hydrophilic coated guide wires are generally avoided during needle access because of the greater ability to create or to enter a dissection plane. In addition, the hydrophilic coating can be “sheared” off by the sharp edge of the entry needle, potentially causing a foreign body embolus.7 When a micropuncture system is used, passage of a hydrophilic stiff wire can be safely performed through the transitional dilator because access to the arterial lumen has already been confirmed. It is crucial to remember that resistance to wire passage is abnormal; it should suggest that the wire is not in the lumen, is in a side-branch vessel, or is in a dissection plane, prompting either fluoroscopic visualization of free tip rotation during advancement or wire removal and confirmation of intravascular placement.
Construction
Guide wires serve the function of negotiating and traversing vessels to facilitate positioning of diagnostic catheters, sheaths, and therapeutic devices. They come in varying diameters, shapes, lengths, flexibility, stiffness, and coatings to facilitate a variety of maneuvers within the various vessels.
The typical access and interventional guide wire is constructed with an outer spring coil welded to an inner member, either a ribbon wire or core wire or both. Guide wires with diameters of more than 0.032 inch are usually constructed with an inner ribbon wire (sometimes called a safety wire) that is welded to both ends of the coil to provide longitudinal integrity. Guide wires with diameters of less than 0.032 inch typically do not have enough room for both the ribbon wire and the core wire. As a result, the core wire only is used, and it is welded at both the proximal and the distal ends to provide longitudinal integrity in the smaller diameter guide wires. Another configuration uses the core wire attached to a hypotube at the end for increased torquability (Fig. 88-4). The core wire material is usually made from stainless steel or nitinol and tapers from the proximal end to the tip. At the tip, the core wire is generally flattened. The core wire affects the torquability of the device; for an ideal wire, the tip of the guide wire will turn in a 1 : 1 ratio with the proximal end. The torquability affects the steerability of the device. Nitinol core wires are more difficult to kink but can lose some torquability. Newer generation small-diameter nitinol wires use a slotted tube design at the end to allow tip shaping. Steel core wires may have varying degrees of ability to shape the tip, depending on the taper or absence of the core at the distal tip. The distal floppy segment of the tip can be varied, and most wires range from 1 to 6 cm. Wires can also be covered with coatings (most commonly polytetrafluoroethylene or silicone) to decrease friction and to increase lubricity. Hydrophilic and hydrophobic tips may be incorporated into the wire, altering the characteristics in the ability to stay within a patent lumen. The Amplatz guide wires use a flat wire versus a more traditional round wire in the manufacture of the coil, thereby reducing the cross-sectional area of the coil in the final guide wire assembly. This design also tends to employ a larger diameter core wire, which provides more body stiffness than can be achieved in a standard round wire coil design of similar diameter.
Diameter
Wire diameter is the first characteristic that can be manipulated in selecting the appropriate platform for the vessel one is negotiating. Size is determined as fractional inches (0.013 inch is equivalent to 1F or one third of a millimeter); 0.035-inch, 0.018-inch, and 0.014-inch wires are the most commonly used sizes in peripheral vascular angiography. The smallest diameter wires are 0.010 inch and are typically used for intracranial neurointerventional procedures. Other diameters uncommonly used include 0.021 inch, 0.025 inch, and 0.038 inch. To improve visibility of smaller wires, the tip may be made with gold, platinum, or palladium, all of which are highly radiopaque. Coronary interventions were once the only procedures done with 0.014-inch wires, but now wires have been designed for peripheral applications as well. The smallest wires (0.010 and 0.0014 inch) are typically described by the tip softness, which can be measured as tip load or the amount of force it requires to bend the tip. Tip loads range from 0.5 to 30 g, with the firmer tips being more desired for crossing of occluded vessels.
Tip Shape
Access and interventional guide wires are also manufactured in both straight and J configurations. The J typically has a 3-mm radius and looks like a shepherd’s hook. The J can be temporarily straightened by pushing forward on the outer wire against the mandrel. This makes for easy insertion into catheters, but it returns to the J shape once it is in the vasculature to help negotiate placement in tortuous vasculature. The most common J radius is 3 mm, but other designs incorporate radii as small as 1.5 mm and as large as 15 mm. Some access wires will incorporate a double distal design with a J tip at one end and a straight tip at the other to facilitate user preference in a single guide wire.
The tip of the guide wire determines the important characteristics of maneuverability and potential vessel injury. J-tip wires cause the least amount of trauma to the vessel, decreasing risk of dissection or perforation. Unfortunately, this feature also makes wires more difficult to steer, to enter small vessel ostia, or to traverse stenoses. Angled, shapeable, or curved tip wires, on the other hand, are valuable for selective cannulation of vessels, with the leading end of the wire used to steer in the desired direction. Spinning the external segment of wire either manually or with the assistance of a torque device translates to the tip direction, allowing precise wire guidance. Straight-tipped wires are used for exchanges or support for devices but are not helpful in vessel selection unless they are used with angled catheters. All guide wires have some length of floppy tip to help minimize vessel injury, most pronounced on starter wires, which have no inner core at the tip. This allows the wire to buckle when it meets resistance, thus decreasing the potential for vessel injury.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



