Chapter 80
Takayasu’s Disease
Natalia O. Glebova, Christopher J. Abularrage
Based on a chapter in the seventh edition by Christopher J. Abularrage and Subodh Arora
Takayasu’s disease is an immune arteritis causing inflammation of the aorta, its major branches, and pulmonary arteries. It is predominantly a disease of young women, with onset typically in the second or third decade of life.1 The disease is more common among Asians, although all ethnicities can be affected. The annual incidence in North America is approximately 2.6 per million people.2
The first description of a patient with Takayasu’s arteritis (TA) may have occurred as early as the 18th century. Morgagni found large-vessel aneurysms and stenoses at autopsy in a 40-year-old woman in 1761.3 In 1830, Yamamoto described a 45-year-old man who originally presented with complaints of fever and developed pulselessness in the radial arteries 1 year later.4 TA is named after Japanese ophthalmologist Mikito Takayasu, who presented his findings of arteriovenous anastomoses of the ocular papilla in a 21-year-old woman with sudden vision loss in 1908.4 Two other ophthalmologists at the same meeting presented similar findings in patients with absent radial pulses.5 Yasuzo Shinmi first coined the term Takayasu’s arteritis in 1939 in describing a patient with comparable symptoms and physical findings.6 In 1975, the research committee of the Japanese Department of Health and Welfare officially proposed adopting the term Takayasu’s arteritis for the disease.
Although TA is a chronic disease, it is characterized by a waxing and waning course. Symptoms range from fever, myalgias, and loss of appetite to cerebral, visceral, and extremity ischemia.7 This variation in presentation frequently leads to a delay in diagnosis.
Epidemiology
TA most commonly affects patients from East Asia, and the incidence varies by geographic location. The estimated incidence in Japan is approximately 150 new cases per year,7 and postmortem studies reveal a prevalence of 1 in 3000 patients.8 The incidence in India is 200 to 300 per million,9 whereas the incidence in Sweden is 0.8 per million, with a prevalence of 0.64 per 100,000.10 In Birmingham, England, the incidence is 0.2 per million.9 Finally, a population-based study in Olmstead County, Minnesota, found an incidence of 2.6 per million.2
TA affects women six to eight times more frequently than men, although this distribution may depend on the region being studied. Authors from India have found female-to-male ratios of 4 : 1,11 whereas others from China have reported ratios of 3 : 1.12 The median age at diagnosis in a cohort of 60 patients from the National Institutes of Health (NIH) was 25 years.13 Although the detection of TA in some patients after the age of 40 years may be related to delays in diagnosis, it is now understood that a significant number of patients do not develop symptoms until later in life. Some authors have suggested that there is a geographic age discrepancy at presentation, with European patients developing symptoms at a later age than their Asian counterparts.10,14 When the results from most major studies are examined, symptoms develop in the majority of patients between the ages of 20 and 40 years, with no trend by region.13,15–20
Patterns of disease have also been described by geographic region. Stenotic lesions are thought to be more common in Japan, whereas aneurysmal disease is more frequent in India, Thailand, and Mexico.7 Patients from Japan and the United States are more likely to have disease of the aortic arch,13,21 whereas patients from India and China more frequently have distal aortic disease.12,22 In a Mexican cohort of 107 patients, 65% had disease throughout the entire aorta.23
Pathogenesis
Etiology
A genetic predisposition to the disease has been suggested because of the predilection for patients of Asian descent, especially those with a Japanese background,24 and the occasional familial clustering of the disease.25 HLA-Bw52 occurs in approximately 44% of Japanese patients with TA compared with 13% in the general Japanese population.26 Further, the haplotype of Bw52-Dw12 confers a greater likelihood of an active inflammatory state and rapid disease progression.27 Other studies have found increased susceptibility in patients with gene mutations in the HLA-A, HLA-D, and HLA-DR regions.28,29 In a group of North American patients with TA, there was no significant association between any HLA antigen and disease severity or complications.30 In fact, there was a negative association between TA and the HLA-DR1 antigen, suggesting a possible protection against disease development. Further studies examining the genetic factors associated with TA must be performed because consistent associations are lacking.31
Immune-mediated mechanisms, including both antibody- and cell-mediated mechanisms, have also been suggested as a cause of TA. TA has been associated with rheumatoid arthritis,32 systemic lupus erythematosus,33 inflammatory bowel disease,34 and glomerulonephritis (see Chapter 78).35 All these conditions have autoimmune mechanisms associated with their pathologic changes, which suggests a common mechanism with TA.
Cell-mediated mechanisms of endothelial damage have been implicated in TA. γδ T cells in patients with TA are reactive to heat shock protein 60 and exhibit cytotoxicity to aortic endothelial cells, suggesting a role in the pathogenesis of TA.36 This mechanism occurs through increased production of interferon-γ and is distinct from other similar rheumatologic disorders.37
Antibody-mediated mechanisms of TA have been advocated on the basis of elevated levels of anti–aortic endothelial cell antibodies. These lead to the induction of endothelial adhesion molecules, cytokines, and apoptosis.38 Levels of anti–aortic endothelial cell antibodies are 20 times greater in patients with TA compared with healthy controls39 and with patients with other autoimmune disorders.40
TA has been associated with tuberculosis in populations in which mycobacteria are endemic.23 On histologic examination, the two may be similar, with granulomatous changes; however, any true association is unlikely because no improvement has been observed after the institution of antituberculosis therapy.
The fact that TA is commonly found in women of childbearing age suggests that sex hormones may play a role in its development. Studies of patients with TA compared with healthy controls have shown elevated urinary estrogens.41 Further, the association of Klinefelter’s syndrome in patients with TA may implicate elevated estrogens in the disease.42
Many causative factors have been implicated in TA, but none is found consistently. The cause of TA is likely to be multifactorial, and further studies are necessary to elucidate the associations among the various suggested mechanisms.
Pathology
Histologic examination of inflammatory lesions of TA reveals a panarteritis of all three layers of the vessel wall, with skip areas along the length of the vessel (Fig. 80-1).43 These lesions can range from acute exudative inflammation to chronic, nonspecific productive inflammation to various types of granulomatous inflammation.44 Active inflammatory lesions begin in the vasa vasorum, extend through the media and adventitia, and terminate in a diffuse or nodular fibrosis. Smooth muscle cells and fibroblasts invade the intima and produce excessive ground substance. On occasion, intimal thickening of the peripheral branches of inflamed arteries may lead to end-organ ischemia.
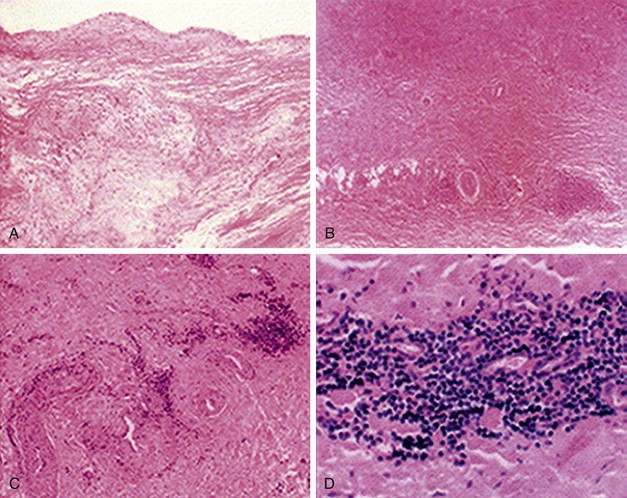
Figure 80-1 Histologic findings in Takayasu’s arteritis. A, Aortic cusp with intimal fibrosis and myxoid degenerative changes in media without inflammatory cells. B, Aortic wall with fibrosis of media and infiltration of inflammatory cells around vasa vasorum. C and D, Inflammatory cell infiltrate consists of predominantly mononuclear lymphocytes. (From Song JK, et al: Echocardiographic and clinical characteristics of aortic regurgitation because of systemic vasculitis. J Am Soc Echocardiogr 16:850-857, 2003.)
Immunohistochemical examination of postoperative aortic specimens with active inflammation has shown replacement of the muscular and elastic layers of the media and adventitia by dense fibrous tissue.45 In certain patients, inflammatory nodules of numerous T cells and B cells co-localized with dendritic cells have been observed in the deep portion of the intima and adventitia. This co-localization suggests that interactions between dendritic cells and lymphocytes may be important in the pathogenesis of TA.
In the chronic phase of TA, inflammation leads to thickening of the entire vessel wall. The vessel lumen narrows owing to proliferation of the intima, destruction of the elastic fibers of the media, and fibrosis of the adventitia.43
Aneurysmal disease may also be associated with TA, although it is less frequent than stenotic disease. It has been hypothesized that aneurysmal disease occurs when destruction of the elastic component of the media occurs before fibrosis of the adventitia, leading to a weakened vessel wall.46
Clinical Presentation
TA was first thought to progress in three phases. The first phase was characterized by an inflammatory period with constitutional symptoms such as fever, headache, weight loss, myalgia, and arthralgia. The second phase was characterized by vessel inflammation with symptoms of vessel pain and tenderness, or carotodynia. The third and final phase, called the burned-out phase, was characterized by vessel fibrosis or aneurysmal degeneration. In this phase, patients presented with the classic signs of ischemia and aneurysm. It is now understood that although most patients with TA experience these three phases, many do not, and patients with TA can endure diverse courses. In a group of North American patients, 10% were asymptomatic at presentation, 57% never had any constitutional symptoms, and only 33% developed systemic symptoms.13 Some TA patients present with inflammatory and fibrotic changes simultaneously, further emphasizing the diverse nature of this disease.7
It is likely that the nonspecific nature of constitutional complaints leads to the delay in diagnosis that is common in patients with TA. We reported a median delay of 1 year before diagnosis in a group of North American patients, similar to the 10 to 15.5 months reported in other studies.13,20 These patients often see multiple physicians for the vague symptoms associated with the disease. Approximately 90% of patients see more than one medical physician, with a mean of five physicians, before obtaining the correct diagnosis of TA.47 A high index of suspicion is appropriate in the usual TA age group because signs and symptoms of vascular insufficiency may be incorrectly perceived as psychosomatic in the absence of physical findings. In fact, up to one third of patients may be inappropriately referred for psychiatric evaluation.
Physical examination may help in diagnosis but is not particularly sensitive. A study examined the association of vascular physical examination findings with arteriographic lesions in patients with established large-vessel vasculitis as part of the Vasculitis Clinical Research Consortium.48 The standardized physical examination included pulse assessment, auscultation for bruits, and measurement of systolic blood pressure differences between extremities. This study revealed that physical examination had good specificity (71%-98%) compared with angiography for detection of lesions in patients with vasculitis. However, the specificity was poor and ranged from 14% to 50%. Thus, normal physical examination findings should not be thought to exclude the presence of arterial disease.
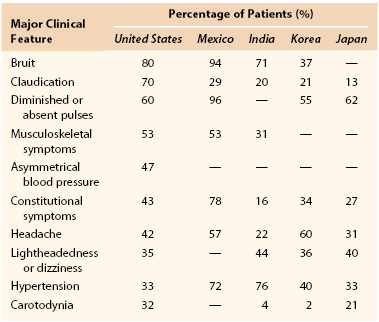
The clinical presentation of patients with TA varies by geographic region (Table 80-1).7 Carotid bruits were the most frequent finding in patients from North America and Italy13,20 versus absent pulses in Mexico49 and hypertension in India.50
Overall, arterial involvement is often symmetrical in paired arteries and contiguous in the aorta.48 Cerebrovascular signs and symptoms are common because of the frequency of aortic arch and branch involvement. Approximately 32% of patients present with carotodynia.13 Many patients report syncope or presyncope at some point during the disease course. Ischemic symptoms of stroke, transient ischemic attack, or amaurosis fugax occur in 5% to 20% of patients.51 Visual disturbances occur in up to 30% of patients and are statistically associated with vertebral and common carotid artery involvement (Fig. 80-2).13 The retinal disease pattern described by Takayasu also leads to visual disturbances due to central retinal hypoperfusion.52
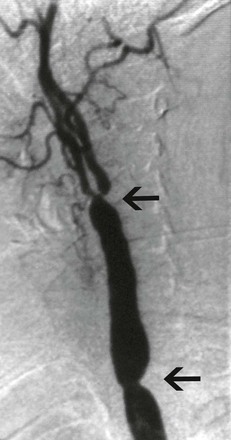
Figure 80-2 Selective carotid angiogram of a 34-year-old woman with symptoms of cerebral ischemia. Note the stenoses of both the common carotid artery and the carotid bifurcation (arrows).
Upper Extremity Involvement
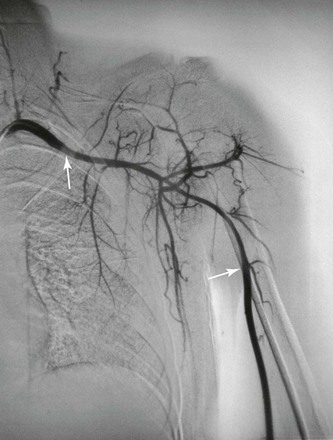
Upper extremity involvement is common in patients with TA. Diminished or absent pulses occur in 53% to 98% of patients.13,49 Effort-induced pain (intermittent claudication) is more frequent in the upper extremities than in the lower extremities and occurs in approximately 62% of patients.13 This may be because the left subclavian artery is more commonly affected by TA than other arteries are (Fig. 80-3).13,20 It has been hypothesized that subclavian steal syndrome is rare in patients with TA because the subclavian artery is involved both proximal and distal to the origin of the vertebral artery. More recent studies have shown that this may not always be true, with angiography demonstrating isolated disease in the proximal subclavian artery 80% of the time.53
Cardiac Involvement
Cardiac manifestations of TA are common. Coronary artery involvement occurs in 6% to 16% of patients with TA and may lead to ischemic symptoms or congestive heart failure.54 Aortic valve regurgitation occurs in 12% to 24% of patients and is generally caused by dilatation of the aortic root.20 Aortic valve regurgitation seems to be more common in TA patients from India.22 Mitral valve regurgitation occurs in 3% to 11% of patients.7,55 The cause of mitral valve regurgitation is unclear but is thought to be independent of aortic regurgitation.56 Pulmonary artery involvement with TA may be underdiagnosed. In studies in which pulmonary angiography was performed, up to 70% of patients had evidence of disease.57,58 These patients most often present with symptoms of pulmonary hypertension. Finally, diffuse vascular involvement of the myocardium has been reported in the absence of coronary stenosis or aortic valve regurgitation. Patients present with symptoms of congestive heart failure, and biopsy specimens of the myocardium show diffuse myocarditis.59
Hypertension
Hypertension is one of the most common presenting signs in patients with TA and occurs in 33% to 60% of patients.12,13 Bilateral stenosis of the subclavian arteries occurs in up to 92% of patients with TA and can mask hypertension.13 Therefore, blood pressure measurements of both upper and lower extremities should be performed in any patient thought to have TA. Renal artery stenosis is a significant cause of hypertension in patients with TA. Involvement of the renal arteries occurs in approximately 20% to 50% of patients.50,60 The stenoses are frequently bilateral and involve the ostia of the vessels (Fig. 80-4).50 Hypertension in the absence of renal artery stenosis occurs in 5% to 30% of patients.13,20 Causes of hypertension in this group of patients include middle aortic syndrome, reduced compliance of the aorta, and dysfunction of the carotid baroceptors (Fig. 80-5).61,62 Sen and colleagues first described 16 patients with middle aortic syndrome in 1963.63 All patients presented with hypertension, and more than half of them had decreased or absent lower extremity pulses. In a 1991 report from the Japanese Ministry of Health and Welfare, middle aortic syndrome occurred in 16% of surgically treated patients with TA.64 Long-segment narrowing of the descending thoracic or abdominal aorta is characteristic, and interscapular or abdominal bruits are frequently heard on auscultation. According to one report, the majority of patients with middle aortic syndrome die by the age of 35 years if they are left untreated.65 Middle aortic syndrome is not unique to TA and may be due to congenital hypoplasia, von Recklinghausen’s disease, fibromuscular dysplasia, and tuberculosis.66–68
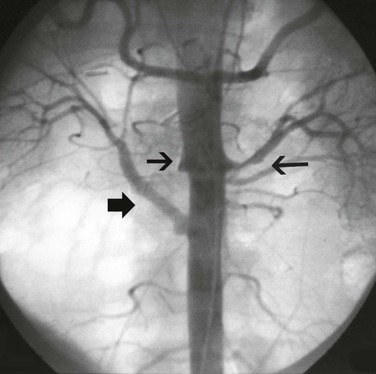
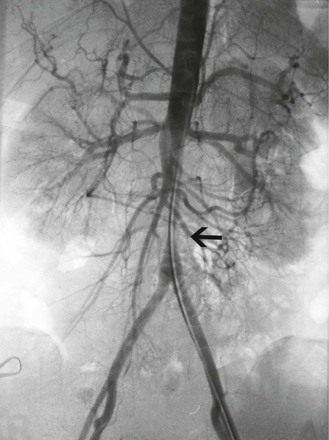
Figure 80-4 Abdominal aortogram of a 48-year-old hypertensive woman with known Takayasu’s arteritis. Note the short-segment left renal artery stenosis (long arrow), right renal artery occlusion (small arrow), and right aortorenal artery bypass (broad arrow).
Visceral Artery Involvement
Visceral arterial involvement occurs in 3% to 31% of patients with TA.20,50 Despite these findings, symptoms of mesenteric ischemia are rare. Aortic endarterectomy for visceral ischemia has been performed, with mixed results. It is controversial in patients with TA because the inflammatory lesions typically involve all three layers of the arterial wall, making the procedure difficult.69,70
Lower Extremity Arterial Involvement
Lower extremity ischemic symptoms occur in 24% to 32% of patients and, as stated previously, are less common than upper extremity symptoms.13,49 Iliac artery disease is seen in approximately 20% of patients undergoing angiography.13,20 Angiographic data suggest that claudication of the lower extremities may be related to abdominal aortic involvement because stenotic disease of the femoral, popliteal, and tibial arteries is rare.20
Aneurysms
Aneurysmal disease in patients with TA varies by geographic location. In South Africa, up to 71% of patients underwent surgery for aneurysmal disease.71 In a study of Thai patients, 60% of patients had aneurysms, with the abdominal aorta being the most common location, followed by the subclavian artery and the thoracic aorta.60 In North America, 27% of patients had aneurysms, with the aortic arch being the most common site, followed by the abdominal aorta (Fig. 80-6).13 Elsewhere, 13.7% of patients in India and 7% of patients in Italy had aneurysms on angiography.20,50 A study from Japan reported 32% of patients with aneurysmal disease and noted that the aneurysms were frequently multiple and associated with stenotic lesions (Fig. 80-7).72 In most series, the incidence of aneurysm rupture is low compared with that of noninflammatory aneurysms.73
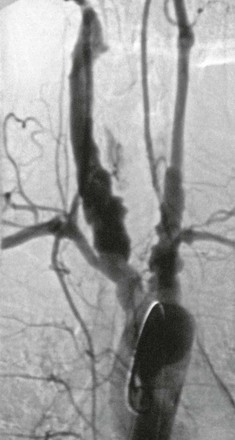
Figure 80-6 Aortic arch angiogram depicting extensive aneurysmal disease of the arch vessels of a 54-year-old man.
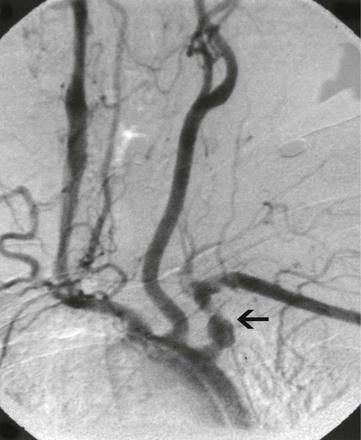
Figure 80-7 Aortic arch angiogram of a 33-year-old woman with left upper extremity claudication. Note the left subclavian aneurysm and concomitant distal stenosis (arrow).
Patients with TA may present with nonischemic symptoms. Raynaud’s phenomenon has been reported in approximately 8% to 14% of patients.20 Skin changes have been reported in 8% to 25% of patients.13,49 These cutaneous manifestations of TA range from erythema nodosum to pyoderma gangrenosum to a lupus-like malar flush.74,75
Diagnostic Evaluation
Diagnostic Criteria
Because TA affects patients in myriad ways, there have been many proposed diagnostic criteria. The first diagnostic criteria, based on a cohort of 96 Japanese patients with TA and 12 patients with other diseases of the aorta, were set forth by Ishikawa.16 These criteria included an obligatory age younger than 40 years. The Ishikawa criteria identified 96% of young patients and 80% of older patients with active disease but, unfortunately, only 67% of young patients and 64% of older patients with inactive disease.
In 1990, the American College of Rheumatology created new diagnostic criteria based on a North American group of patients with TA (Table 80-2).76 It removed Ishikawa’s age criterion because the disease developed in 13% of patients after the age of 40 years.13 The presence of at least three of the six criteria from the traditional classification demonstrated a sensitivity of 90.5% and a specificity of 97.8%. The same group also created a classification tree with five of these six criteria, omitting claudication of an extremity. The classification tree demonstrated a sensitivity of 92.1% and a specificity of 97.0%.
Table 80-2
American College of Rheumatology 1990 Diagnostic Criteria for Takayasu’s Arteritis
| Criteria | Definition |
| Age at disease onset in years | Symptoms or findings of Takayasu’s arteritis at <40 years |
| Extremity claudication | Lower or upper extremity muscle fatigue during exercise |
| Diminished brachial artery pulse | Unilateral or bilateral |
| Blood pressure difference >10 mm Hg | Measured systolic blood pressure between upper extremities |
| Bruit over subclavian arteries or aorta | One or both subclavian arteries or aorta |
| Angiographic abnormalities | Narrowing or occlusion of the aorta, primary branches, or large arteries in proximal extremities; must not be secondary to atherosclerosis, fibromuscular dysplasia, or other causes; usually focal or segmental |
Modified from Arend WP, et al: The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 33:1129-1134, 1990.
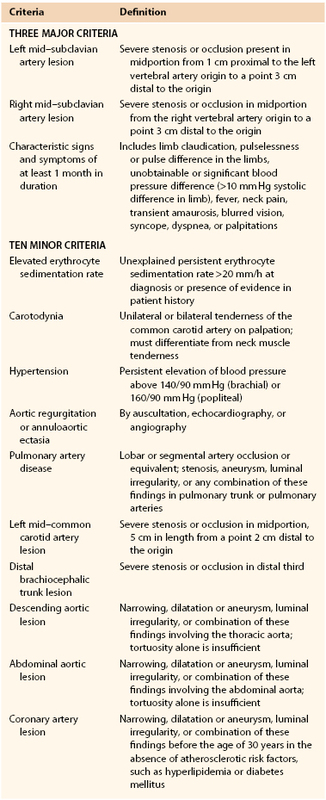
In 1996, Sharma and associates devised a third set of diagnostic criteria. The Ishikawa criteria were modified in an effort to improve early diagnosis and to take into account geographic and racial variations (Table 80-3).77,78 The presence of two major criteria, one major criterion and two minor criteria, or four minor criteria was found to have a sensitivity of 92.5% and a specificity of 95% in a group of Indian patients and 96% sensitivity and 96% specificity in a group of Japanese patients.
Although there is no global consensus on the best diagnostic criteria, it is generally agreed that the diagnosis of TA should not be made without ruling out all other vasculitides, vascular infections, fibromuscular dysplasia, and idiopathic inflammatory syndromes.79
Laboratory Markers
TA is not associated with any specific laboratory abnormality but is characterized by generalized inflammatory markers. Patients are frequently anemic, with hematocrits ranging from 20% to 30%.43 The erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are the most common markers used for diagnostic purposes. At presentation, approximately 72% of patients with TA have an elevated ESR.7 The ESR is nonspecific, however; 56% of patients have an elevated ESR with disease remission, and 28% have a normal ESR with clinically active disease.
Investigators have begun to examine the role of other laboratory markers in TA, including serum interleukin-6 (IL-6) and the factor named regulated upon activation, normal T-cell expressed, and presumably secreted (RANTES). IL-6 enhances T-cell cytotoxicity and natural killer cell activity80,81; RANTES is a selective chemoattractant for most mononuclear cell types implicated in TA.82 A study showed increased levels of IL-6 and RANTES in patients with TA.83 Furthermore, levels of both IL-6 and RANTES paralleled disease activity.
Other investigators have examined matrix metalloproteinases (MMPs) in patients with TA.84 MMP-2 levels were elevated in patients with TA compared with controls, although no correlation was found between serum MMP-2 and disease activity. MMP-3 and MMP-9 levels in patients with active disease were elevated compared with those in controls and patients in disease remission, and a positive correlation was found between MMP-3 or MMP-9 level and disease activity.
Other markers being examined include IL-8,85 IL-18,86 and tumor necrosis factor-α (TNF-α).87 At present, there is no single serologic test for TA, and the role of specific serum markers is still being delineated.
Radiologic Evaluation
Although histopathologic examination of arterial specimens provides the most accurate determination of disease, radiologic evaluation can provide a more extensive assessment of the pathologic process. In the past, digital subtraction angiography of all possibly affected arteries was considered the only reliable radiologic method of evaluating patients with TA.88
More recently, noninvasive methods such as ultrasound, computed tomographic angiography (CTA), and magnetic resonance angiography (MRA) have been used to assess the mural changes of the arterial wall seen in TA before the development of stenotic or aneurysmal disease.89 Duplex ultrasound has been useful for evaluating the carotid arteries in patients with TA. Active disease is characterized by prominent wall thickening with a maintained outer diameter, whereas inactive disease is characterized by mild wall thickening with a decreased outer diameter (Fig. 80-8).90 A comparison of duplex ultrasound and arteriography of the carotid arteries reveals that the two closely correlate in terms of disease assessment and that ultrasonographic mural thickness is a more sensitive indicator of early, latent inflammation.91
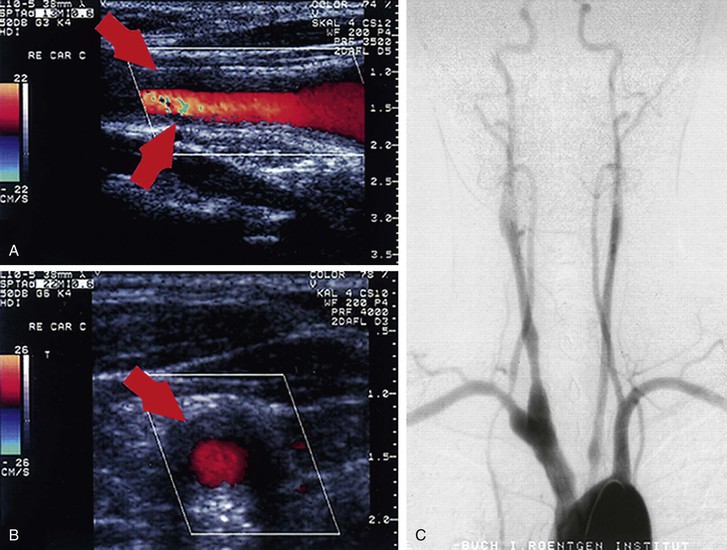
Figure 80-8 Patient with early Takayasu’s arteritis. A and B, Ultrasound images (A, longitudinal plane; B, transverse plane) of the right common carotid artery with the typical morphologic appearance of Takayasu’s arteritis indicated by arrows. C, Angiography of the branches of the aortic arch, confirming the ultrasound diagnosis and revealing narrowing of up to 40% as well as post-stenotic dilatations at the right common carotid artery, the left proximal common carotid artery, the proximal right subclavian artery, and the proximal right vertebral artery. (From Schmidt WA, et al: Diagnosis of early Takayasu arteritis with sonography. Rheumatology (Oxford) 41:496-502, 2002.)
Computed Tomography Angiography
Findings on CTA may include high density and calcifications of the aortic wall on precontrast images, a thickened wall with enhancements in the arterial and venous phases, and a low-attenuation ring in the venous phase (Fig. 80-9).92 CTA is also useful to assess the extent of disease involvement. In one study, the extent assessed by mural change was wider than that assessed by luminal change in 61% of patients.93 Skip lesions were seen in 16% of patients, although contiguous arterial involvement was more common (81%). Finally, CTA revealed the coexistence of active and inactive lesions in 11% of patients. A comparison of CTA with conventional angiography showed that the two correlated in 71% of patients, but CTA depicted more extensive disease owing to its ability to assess mural inflammation.94
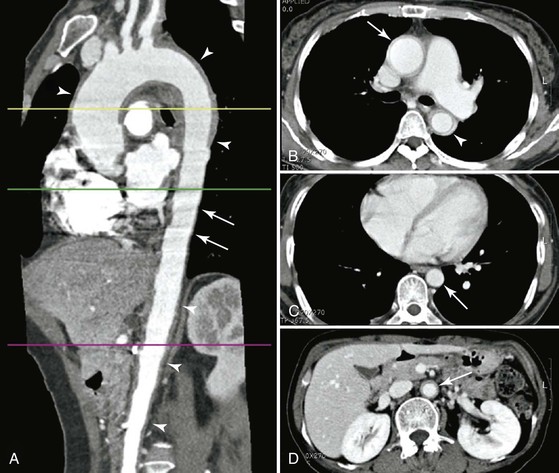
Figure 80-9 Takayasu’s arteritis involving the aorta with a skipped segment in a 50-year-old woman. A, Multiplanar reformation image shows wall thickening (arrowheads) of ascending thoracic aorta, aortic arch, proximal descending thoracic aorta, and abdominal aorta. No wall thickening of distal descending thoracic aorta is noted. Motion artifact (pulsation artifact) was not observed in the thickened segment, such as the aortic arch and abdominal aorta, because of stiffness of the involved aorta but was observed in the distal descending thoracic aorta (arrows) owing to pulsation of the noninvolved segment. B, CT scan at the proximal descending thoracic aorta shows diffuse wall thickening and inner low-attenuation ring of ascending (arrow) and descending thoracic aorta (arrowhead). C, CT scan at the distal descending thoracic aorta (arrow) shows no wall thickening of aorta. D, CT scan at the abdominal aorta shows diffuse wall thickening and inner low-attenuation ring of abdominal aorta (arrow). (From Chung JW, et al: Patterns of aortic involvement in Takayasu arteritis and its clinical implications: evaluation with spiral computed tomography angiography. J Vasc Surg 45:906-914, 2007.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree