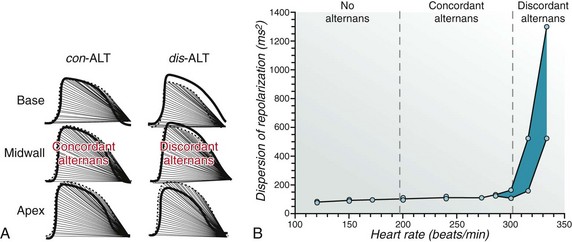
69 Electrical alternans is defined as beat-to-beat alterations in the shape of electrocardiographic waveforms. The occurrence of visible electrical alternans (i.e., “macrovolt” alternans) was first described by Hering in 1908.1 Shortly thereafter, Thomas Lewis noted that alternans could occur in a normal heart following marked accelerations in heart rate and also in the diseased or intoxicated myocardium.2 In 1948 Kalter and Schwartz examined the electrocardiograms (ECGs) from 6059 patients and described an association between macroscopic T wave alternans (TWA) (observed in five patients) and an increased mortality of affected patients.3 Subsequent case reports described the occurrence of visible TWA in various clinical situations such as myocardial ischemia, coronary artery spasm, and electrolyte disturbances, particularly in the setting of congenital long QT syndrome.4 Assessment of subtle microvolt TWA (MTWA) through sophisticated computerized analysis was first reported in 1982.5 In the 1980s Cohen and coworkers established a close relationship between MTWA and vulnerability to ventricular fibrillation (VF) in a dog model.5,6 Similar findings were subsequently reported by Nearing et al.7 Since the inscription of the methods for assessment of MTWA, compelling evidence for a mechanistic link between MTWA and the occurrence of ventricular tachyarrhythmias has been provided in a plethora of experimental and clinical studies. Clinical studies using implantable cardioverter-defibrillator (ICD)-stored electrogram series8 or recordings from ambulatory monitoring9 have demonstrated a progressive increase in MTWA shortly before the onset of ventricular tachyarrhythmias. MTWA results from beat-to-beat alternation in the time course of the action potential duration at the cellular level. This was definitively established through detailed measurements of the time course of membrane voltage throughout the ventricle at a time when MTWA was elicited on the surface ECG using high-resolution optical mapping techniques in a guinea pig model of pacing-induced MTWA.10–12 In these studies MTWA was a consistent (in fact requisite) precursor to ventricular fibrillation. The mechanism linking action potential alternans (and therefore MTWA) to ventricular arrhythmias involves the development of spatially discordant alternans (i.e., action potential alternans) occurring with opposite phase between neighboring cells (Figure 69-1, panel A).10 When action potential alternans is initiated, action potential duration either prolongs or shortens simultaneously in all cells of a particular region of ventricular myocardium (i.e., concordant alternans; Figure 69-2, panel A, left). As illustrated in Figure 69-2 (panel A, right), after a premature impulse (*) or change in pacing rate above a critical heart rate threshold, sequential lengthening and shortening of the action potential occurs, with fluctuations in some regions being 180 degrees out of phase with those in other regions, such that some cells undergo a prolongation of action potential duration while other populations of cells undergo action potential duration shortening on the same beat (i.e., discordant alternans).10,12 During discordant alternans (see Figure 69-2, panel B, right), marked spatial dispersion of action potential duration emerges (blue bars), and discordant alternans amplifies physiological heterogeneities of repolarization present at baseline into pathophysiological heterogeneities of sufficient magnitude to produce conduction block and reentrant excitation (Figure 69-2, panel B).10–12 Discordant alternans also produces a substrate by which conduction block and reentrant excitation can be easily initiated by a premature stimulus (see Figure 69-2, panel B). When an impulse (*) propagates (from site A) into still depolarized myocardium (e.g., in the wake of enhanced dispersion of repolarization after the long beat; blue bar at site B), conduction block initiating reentrant excitation can occur (see Figure 69-2, panel B; VF). Consequently, discordant alternans is a mechanism linking MTWA to cardiac arrhythmogenesis, and, in fact, in experimental models of action potential alternans, ventricular fibrillation never occurs without discordant alternans. Thus, under chronotropic or metabolic stress, discordant alternans leads to sufficiently large repolarization gradients to produce unidirectional block and reentry. Without a structural barrier, reentry is functional and manifests as ventricular fibrillation or polymorphic ventricular tachycardia. In the presence of structural barriers, reentry can become anatomically fixed, resulting in monomorphic ventricular tachycardia. Figure 69-1 Left side, A, Action potential assessed by high-resolution optical mapping from epicardial cells on base, apex, and mid-wall of the guinea pig left ventricle during two consecutive beats. Above a critical heart rate, concordant alternans is transformed to discordant alternans between apex and base. Right side, B, Amplification of spatial gradients of repolarization by discordant alternans. con-ALT, Concordant alternans; dis-ALT, discordant alternans. (Adopted from Pastore JM, Girouard SD, Laurita KR, et al: Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation 99:1385-1394, 1999.) Figure 69-2 Concordant and Discordant Alternans and the Induction of Ventricular Fibrillation Although the cellular mechanism for action potential alternans is not completely elucidated, convincing data point to a primary role of sarcoplasmic reticulum calcium cycling in its mechanism.13 Merchant and Armoundas recently provided an in-depth review of the cellular mechanisms of alternans.14 In essence, they emphasize two major hypotheses to explain alternans at the cellular level. The first hypothesis postulates that alternation in sarcolemmal currents, membrane voltage, and action potential morphology results in beat-to-beat fluctuations in intracellular calcium concentration. For instance, Shimizu and Antzelevitch used a ventricular wedge preparation in which congenital long QT syndrome physiology was mimicked.15 With this preparation, alteration of the T wave and action potential duration was elicited during rapid pacing; application of ryanodine or lowering of extracellular calcium concentration abolished these alterations, implicating intracellular calcium cycling in the maintenance of MTWA. The second major hypothesis suggests that alternation of intracellular calcium concentration is the initial event leading during the second step to alternans of membrane voltage and action potential morphology.10,11,16,17 According to this second hypothesis, alternans of intracellular calcium concentration may be caused by perturbations of any number of calcium transport processes that may occur in diseased myocardium.14 A wealth of recent experimental data support the second hypothesis, indicating that perturbations of calcium handling are the fundamental event in the genesis of cellular alternans. Two contemporary techniques for detection and quantification of MTWA have been developed and applied in clinical studies of risk stratification: the spectral method6 and the modified moving average (MMA) method.7 The spectral method uses the vector magnitude ECG signal recorded from the three frank orthogonal leads over at least 128 beats (Figure 69-3). Each T wave is measured at the same time relative to the QRS complex. Because this spectrum is created by measurements taken once per beat, its frequencies are reported in the unit of cycles per beat. Accordingly, the point on the spectrum corresponding to 0.5 cycles per beat indicates the level of alteration of the T wave wavefront (see Figure 69-3). The alternans power (µV2) is defined as the difference between the power of the alternans frequency and the power at the noise frequency band (calculated over the reference frequency band between 0.44 and 0.49 cycles per beat). This is a measure of the true physiological alternans level. The alternans voltage (Valt) is simply the square root of alternans power and corresponds to the root mean square difference in the voltage (averaged over the T wave) between the overall mean beats and either the even-numbered or odd-numbered mean beats. A measure of the statistical significance of the alternans is defined as the alternans ratio (K score), calculated as the ratio of the alternans power divided by the standard deviation of the noise in the reference frequency band. Alternans is considered significant if the K score is ≥3. This spectral method has been demonstrated to provide a robust measurement of MTWA. Figure 69-3 Schematic Representation of MTWA Assessment Using the Spectral Method Determination of MTWA requires increases in heart rate. For every patient, there is a threshold heart rate, above which MTWA will become apparent (see later). In the initial studies, heart rate was increased by means of atrial pacing.18–20 Subsequently, techniques were developed to noninvasively elevate heart rate by means of treadmill or bicycle exercise testing. MTWA test results obtained with both methods were highly reproducible.19 Currently, MTWA assessment is uniformly performed noninvasively. As with every evolving new method, classification schemes for the assessment of MTWA had to be developed on the basis of results of early clinical studies. Accordingly, a detailed synopsis on current guidelines for interpretation of MTWA tests has been published.21 In brief, the presence of MTWA is defined by its magnitude, the alternans ratio (K score), the relationship between MTWA and heart rate, and the assessment of the presence of artifacts that can cause alternans. Significant MTWA is defined as alternans having Valt ≥1.9 µV with a K score ≥3. Sustained alternans is defined as significant alternans that is at least 1 minute in duration and is consistently present above a patient-specific threshold heart rate, which is referred to as the onset heart rate.19 Once the onset heart rate has been reached, patients consistently have alternans until their heart rate falls below that threshold. • Positive tracings. If sustained alternans is present with an onset heart rate ≤110 beats/min (bpm), then the tracing is classified as positive. The choice of the threshold heart rate of 110 bpm is based on the observation that normal healthy subjects may develop alternans at high heart rates, suggesting that alternans at high heart rates is not prognostically significant. If a tracing has sustained alternans but the onset heart rate is above 110 bpm, then the tracing will be negative or indeterminate according to the patient’s maximum negative heart rate (Figure 69-4). • Negative versus indeterminate tracings. The distinction between tracings that are negative and those that are indeterminate is based on the maximum negative heart rate—the highest heart rate on the tracing in which one is confident that alternans is not present. The original classification used prospectively in a number of studies22,23 required that the maximum negative heart rate must be ≥105 bpm for a tracing to be classified as negative. For example, if a tracing has excessive levels of ectopy or noise above a heart rate of 95 bpm, one cannot be confident that alternans is not present above a heart rate of 95 bpm, and one cannot consider the tracing to be negative. Accordingly, the maximum negative heart rate is 95 bpm and the tracing is classified as indeterminate. Perhaps if the ectopy or noise levels were reduced, alternans would be “unmasked” at a heart rate of 100 bpm (Figure 69-4). For this reason, tracings without sustained alternans and with a maximum negative heart rate <105 bpm are considered indeterminate. Similarly, tracings with sustained alternans with an onset heart rate >110 bpm (i.e., alternans that is not prognostically significant) are also classified as indeterminate if the maximum negative heart rate is <105 bpm. Figure 69-4 Examples of MTWA Positive, Negative, and Indeterminate Test Results Application of this classification can result in indeterminate results in up to 25% to 30% of some populations.22–24 Many tracings were classified as indeterminate because the patient’s maximal heart rate was less than 105 bpm, and therefore the tracing had no chance of being classified as negative (the maximum negative heart rate cannot be greater than the maximum heart rate). MMA-based MTWA can be assessed during exercise stress testing, during postexercise recovery, or on the basis of ambulatory ECG recordings.25 MMA is based on the noise-rejection principle of recursive averaging.26 As shown in Figure 69-5, the algorithm continuously streams odd and even beats into separate bins and creates median complexes for each bin.7 These complexes are then superimposed, and the maximum difference between odd and even median complexes at any point within the JT segment is determined as the TWA value, which is averaged every 10 to 15 seconds. The moving average allows control of the influence of new incoming beats on the median templates with an adjustable update factor, that is, the fraction of morphology change that an incoming beat can contribute. Respiration and motion artifacts are reduced by noise-reduction software. MMA-based MTWA can be assessed noninvasively from routine, symptom-limited exercise stress testing and ambulatory ECG monitoring using precordial ECG leads with standard electrodes. Careful skin preparation including mild abrasion is necessary for maximal signal-to-noise ratio. The algorithm excludes extrasystoles, noisy beats, and the beats preceding them. Risk stratification is based on the peak MTWA value throughout the 24-hour ECG recording or the exercise test. Using the MMA method, MTWA ≥60 µV during routine exercise testing or ambulatory ECG monitoring25,27,28 was found in clinical studies to be associated with increased risk for sudden cardiac death and/or cardiovascular mortality. In patients during the early post–myocardial infarction (MI) phase with or without heart failure, a lower cutpoint of ≥47 µV predicted sudden cardiac death.29,30 Leino et al.31 demonstrated 55% and 58% increases in risk of cardiovascular and sudden cardiac death, respectively, per 20 µV of TWA.
T Wave Alternans*
History of Cardiac Alternans
Mechanisms Underlying T Wave Alternans
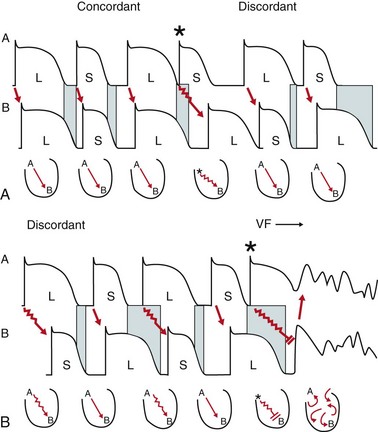
Upper panel, Transition of concordant to discordant alternans after a premature impulse (asterisk). Lower panel, Occurrence of conduction block and reentrant excitation leading to ventricular fibrillation (see text for details). (Adopted from Wilson LD, Rosenbaum DS: Mechanisms of arrhythmogenic discordant cardiac alternans. Europace 9[Suppl 6]:77-82, 2007.)
Cellular Mechanisms of Alternans
Methodology of TWA Assessment
The Spectral Method
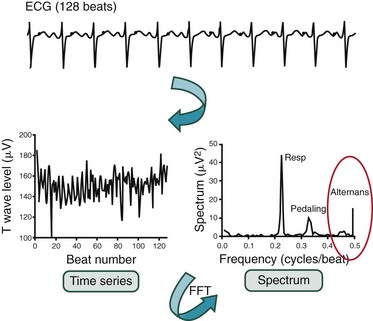
The amplitudes of the corresponding points on the T wave are measured for 128 beats. A time series consisting of these 128 amplitudes is created. The power spectrum of this time series is computed using fast Fourier transform methods. In the power spectrum obtained from recordings during bicycle exercise, peaks corresponding to frequencies of respiration, pedaling, and alternans are illustrated. Microvolt TWA appears as a peak at exactly one-half the beat frequency (0.5 cycles per beat). The amplitude of this peak is compared with the mean and standard deviation of the spectrum in a reference “noise band.” FFT, Fast Fourier transformation. (Adopted from Cohen RJ: TWA and laplacian imaging. In Zipes DP, Jalife P [eds]: Cardiac Electrophysiology: From Cell to Bedside, 3rd ed. Philadelphia, Saunders, 1999, pp 781-789.)
Classification and Interpretation of MTWA Using Spectral Analysis
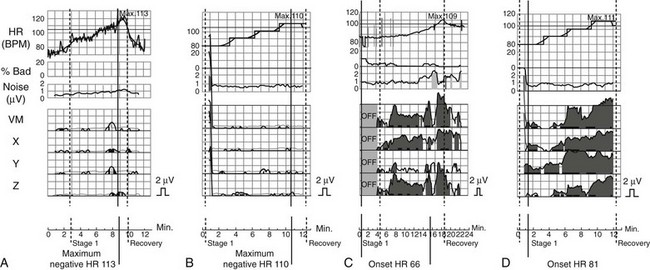
Shown (from top to bottom) are heart rate trend; percentage of bad beats; noise level; and MTWA amplitudes in vector magnitude lead VM, orthogonal leads X, Y, and Z, and ECG leads V1 to V6. A, Example for a bicycle exercise negative MTWA test. B, Atrial pacing test in the same patient is also negative for MTWA. C, Example of bicycle exercise-induced sustained MTWA (gray shaded area). D, Atrial pacing test in the same patient yields also MTWA positive findings.
The Modified Moving Average Method
Classification and Interpretation of MTWA Using the MMA Technique
T Wave Alternans