HF remains one of the most common reasons for hospitalization in the United States, particularly in elderly patients. Hospital discharges for HF increased from 877,000 in 1996 to over 1.1 million in 2006. In contrast, the number of ambulatory visits for HF in 2007 was approximately 3.4 million. The estimated direct and indirect cost of HF in the USA for 2010 is $39.2 billion.
12.3 MORTALITY
While the rate of death after HF onset declined in both sexes by approximately one-third from the 1950s to the 1990s, mortality has not significantly changed over the past two decades. Data from the Framingham Heart Study, which evaluated participants and their offspring, revealed that 80% of males and 70% of females with HF who are younger than 65 will die within eight years. Once HF is diagnosed, females tend to survive longer than males, although less than 15% of females survive more than 8–12 years. The one-year mortality rate after HF diagnosis is estimated at 20%. Patients with heart failure are 6–9 times more likely to die from sudden cardiac arrest compared to the general population.
12.4 PATHOPHYSIOLOGY OF LV SYSTOLIC DYSFUNCTION
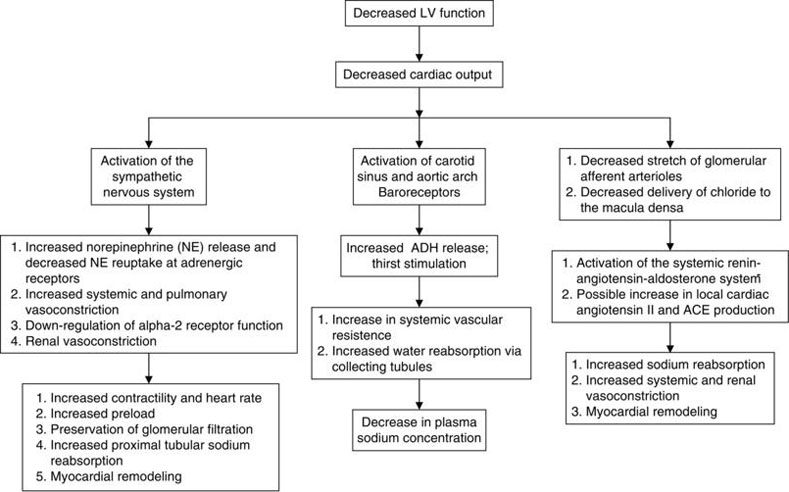
Cardiac output is the product of stroke volume and heart rate. In LV systolic dysfunction, there is a decrease in myocardial contractility which leads to a decrease in stroke volume, and therefore, cardiac output (Figure 12.1). Associated cardiac remodeling further impairs cardiac function. The body’s initial response to decreased cardiac output is increased sympathetic activity which restores cardiac output by increasing heart rate and contractility. Decreased cardiac output also promotes salt and water retention by the kidney via the renin-angiotensin-aldosterone system (RAAS). While these mechanisms initially compensate for the decrease in LV contractility and allow for sufficient end-organ perfusion, they eventually lead to maladaptive consequences including peripheral vasoconstriction, stimulation of myocardial fibrosis, and worsened cardiac remodeling. Adverse cardiac remodeling impairs wall stress and leads to an increase in myocardial oxygen demand. For many, the vicious cycle of RAAS activation and fibrosis leads to end-stage cardiac failure that is recalcitrant to standard medical therapy.
12.5 CAUSES AND RISK FACTORS
Hypertension (HTN), diabetes mellitus (DM), and previous myocardial infarction (MI) are the most common risk factors associated with HF in patients with an elevated BMI or a decreased creatinine clearance. Other risk factors include valvular disease, macro- or microvascular coronary artery disease, tobacco use, toxin exposure (alcohol, chemotherapy), viral infection, poorly controlled atrial arrhythmias (“tachycardia-induced cardiomyopathy”) and thyroid disease (Table 12.2).
Table 12.2 Risk Factors for the Development of HF.
| Hypertension (RR 1.4, PAR 10%) Coronary heart disease (RR 8.1, overall PAR 62%) Cigarette smoking (RR 1.6, PAR 17%) Overweight (RR 1.3, PAR 8%) Diabetes mellitus (RR 1.9, PAR 3%) Valvular heart disease (RR 1.5, PAR 2%; this risk factor increases with age) |
PAR, Population Attributable Risk (estimates the proportion of cases that could be prevented if the risk factor was eliminated from the population); RR, Relative Risk.
12.6 CLINICAL HISTORY AND PHYSICAL EXAMINATION
Patients with decompensated HF often have a history of worsened dyspnea, fatigue, orthopnea, and PND. Exam often reveals rales, cardiac wheezing, worsened hypoxia or edema (of the legs and/or abdomen), the presence of jugular venous distention, and an S3 on cardiac exam. However, it is important to realize that not all patients with dyspnea and/or volume overload have HF. Other diagnoses that should always be considered include COPD exacerbation, pneumonia, PE, pulmonary hypertension, ischemic heart disease, arrhythmia, liver, and renal disease. Labwork and studies should help elucidate the underlying causative process (although in some cases it may be multifactorial). Brain Naturietic Peptide (BNP) has proven to be useful in distinguishing HF from other potential causes of dyspnea. A level of 400 or greater has a high positive predictive value for HF, whereas a level less than 100 has a high negative predictive value. Levels between 100 and 400 are neither sensitive nor specific for excluding HF. BNP levels tend to be higher in older individuals, women, and those with renal insufficiency; they tend to be lower in obese patients. EKG helps evaluate for evidence of arrhythmia, ischemia, interval infarction, right heart strain, and PE. Chest X-ray may show evidence of HF: cardiomegaly, pulmonary edema, or pleural effusions. Alternatively, it may show evidence of another process such as COPD or pneumonia.
12.7 OUTPATIENT MEDICAL MANAGEMENT
In addition to lifestyle modification and management of underlying etiologies and risk factors, medical therapy improves morbidity and mortality in patients with systolic heart failure. Therapies with proven morbidity and mortality benefit include ACE inhibitors (or ARBs if the former are not tolerated), beta-blockers, aldosterone antagonists, and hydralazine/nitrate combination therapy (Table 12.3). In addition, diuretics and digoxin may be considered for symptom improvement.
12.8 IMPLANTABLE CARIOVERTER-DEFIBRILLATOR (ICD) ± CARDIAC RESYNCHRONIZATION THERAPY
ICD implantation is indicated for primary and secondary prevention of sudden cardiac death in heart failure patients with a history of documented ventricular fibrillation (VF), hemodynamically unstable ventricular tachycardia (VT), VT with syncope, and LVEF less than or equal to 35% with significant HF symptoms. Table 12.4 shows guidelines for ICD implantation in patients with HF. A systemic review published in 2007 found that ICD implantation reduced all-cause mortality in adult patients with LV systolic dysfunction, most of whom had class II or III NYHA symptoms. Among randomized controlled trials this reduction was found to be 20% (largely due to a relative reduction in sudden cardiac death); in observational studies this reduction was found to be 46%.
Table 12.3 Medications Used in HF to Improve Morbidity and Mortality.
| ACE inhibitors |
| Improve morbidity and mortality in all HF classes Trials: CONSENSUS, SOLVD, XSOLVD, SAVE May not be as effective in women and African Americans (V-HeFT) Start with enalapril 2.5 mg bid, captopril 6.25 mg tid, lisinopril 2.5–5 mg daily Goal enalapril 20 mg bid, captopril 50 mg tid, lisinopril 40 mg daily Surveillance labs including serum BUN/creatinine and potassium should be checked 3–7 days after drug initiation, during titration and after goal dose has been reached Side effects: hypotension, worsened renal function, hyperkalemia, cough, angioedema |
| Beta-blockers |