Neointimal hyperplasia after percutaneous coronary intervention is a major determinant of in-stent restenosis (ISR). Drug-eluting stents (DES) mitigate neointimal hyperplasia and thereby lead to a lower rate of ISR compared with bare-metal stents (BMS). Recent studies have demonstrated that short-term use of oral sirolimus after BMS leads to a significant reduction in ISR. We therefore sought to do a systematic review of studies to determine the angiographic and clinical benefits of early short-term use of oral sirolimus after BMS of native coronary arteries. We conducted PubMed, Embase, Cochrane database review, and Web of Science search of studies comparing oral sirolimus after BMS to BMS alone or DES. Outcomes analyzed were ISR and target lesion revascularization (TLR) as well as major adverse cardiovascular events. A total of 488 patients from 4 studies were included in the review (2006 to 2010). Three studies, comparing BMS alone versus BMS plus oral sirolimus, demonstrated significant reduction in ISR in the oral sirolimus group. Two of these studies also demonstrated significant reduction in TLR at 6-12 month follow-up. The fourth study comparing BMS plus oral sirolimus versus DES showed a lower but nonsignificant reduction in TLR in addition to significant cost saving in the group treated with oral sirolimus. In conclusion, our systematic review demonstrates that early short-term systemic use of sirolimus after BMS resulted in a significant reduction in ISR and TLR. In addition, ISR rates were comparable to DES with the added benefit of cost saving.
Neointimal hyperplasia precipitated by acute vessel injury is mediated by vascular smooth muscle cell proliferation, migration to the subintimal layers, and synthesis of extracellular matrix. In-stent restenosis (ISR) is highly prevalent after bare-metal stents (BMS). Drug-eluting stents (DES) have dramatically reduced ISR. Randomized clinical trials have systematically shown a reduction in need for repeat revascularization with DES. Nonetheless, despite great enthusiasm over the use of DES, several potential concerns still remain, prompting a need for alternative therapies that would decrease the need for prolonged dual antiplatelet therapy (DAPT) without compromising target lesion revascularization (TLR). Systemic use of oral sirolimus has been associated with a significant reduction in intimal hyperplasia in animal models. Clinical studies to date have also assessed the utility of oral sirolimus after BMS with a hypothesis that such therapy would lead to a reduction in neointimal hyperplasia, ISR and hence a reduction in repeat revascularization. We hereby present a systematic review of the studies on the early short-term use of oral sirolimus after BMS.
Methods
A systematic review of the studies assessing the efficacy of the oral administration of sirolimus after percutaneous coronary intervention using BMS was conducted. We searched PubMed, Web of Science, Cochrane, and Embase databases with the following combination of terms: coronary stent OR percutaneous coronary intervention AND oral Sirolimus OR oral Sirolimus. We included all studies comparing BMS or DES with BMS plus oral sirolimus. All studies were retrieved in full forms and analyzed independently by investigators for appropriate inclusion in the systematic review.
Each study was carefully analyzed for methodology, inclusion and exclusion criteria, angiographic methods, follow-up, and outcomes. Once investigators independently reviewed all studies, the available data were collected and formulated in tabulated forms for analysis. The primary end point in our analysis was the rate of binary ISR defined as a binary stenosis of ≥50% of the lumen diameter. The secondary end points were TLR, target vessel revascularization, and major adverse cardiovascular events including myocardial infarction and cardiac death.
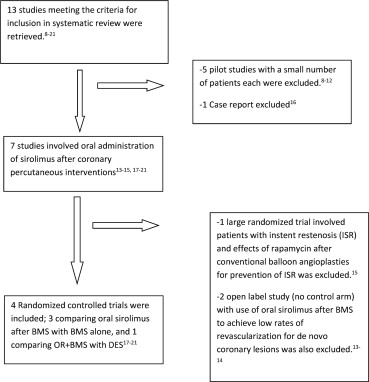
Our search of studies yielded a total of 13 studies ( Figure 1 ). Five pilot studies were excluded. Two studies with no control arm were excluded. One study was excluded as patients with ISR were included at baseline. One case report was excluded. Thus, the final analysis included 4 randomized controlled trials; 3 of which compared BMS versus BMS plus oral sirolimus and 1 comparing BMS plus oral sirolimus versus DES.
Results
A total number of 488 patients from 4 studies (2006 to 2010) were included in this analysis. Baseline characteristics are listed in Table 1 . The mean age of the patients was 62 years, and most were men. Hypertension and hyperlipidemia were highly prevalent. Left anterior descending artery was the most commonly treated vessel. According to the American College of Cardiology/American Heart Association lesion classification scheme, most of the lesions treated across the 4 studies were B1 and B2.
| Characteristic | Rodriguez et al | Stojkovic et al | Cernigliaro et al | Rodriguez et al | ||||
|---|---|---|---|---|---|---|---|---|
| Oral Sirolimus + BMS Group (%) | Control Group (%) | Oral Sirolimus + BMS Group (%) | Control Group (%) | Oral Sirolimus + BMS Group (%) | Placebo + BMS Group (%) | Oral Sirolimus + BMS Group (%) | DES Group (%) | |
| Number of patients | 50 | 50 | 40 | 40 | 54 | 54 | 100 | 100 |
| Age (yrs) | 64.6 ± 9 | 65.1 ± 8 | 58.9 ± 10 | 56.8 ± 9 | 63.9 ± 10 | 62.1 ± 10 | 62.1 ± 10 | 63.4 ± 10 |
| Men | 88 | 94 | 67 | 73 | 85 | 83 | 83 | 81 |
| Diabetes | 24 | 8 | 28 | 30 | 19 | 17 | 24 | 33 |
| Hyperlipidemia | 92 | 92 | 73 | 63 | 44 | 39 | 71 | 81 |
| Hypertension | 92 | 82 | 70 | 68 | 56 | 50 | 69 | 72 |
| Smoking | 24 | 18 | 75 | 78 | 35 | 37 | 21 | 17 |
| Previous myocardial infarction | 22 | 38 | 58 | 68 | 43 | 46 | 26 | 33 |
| Stable angina | 0 | 2 | 35 | 40 | 24 | 20 | — | — |
| Unstable angina | 76 | 54 | 58 | 50 | 24 | 22 | 62 | 56 |
| Target artery | ||||||||
| Right | 20 | 27 | 28 | 27 | 27 | 21 | 20 | 23 |
| Left anterior descending | 53 | 40 | 42 | 34 | 48 | 52 | 45 | 48 |
| Left circumflex | 25 | 33 | 30 | 39 | 23 | 26 | 32 | 26 |
| Left main | 2 | 0 | — | — | 2 | 1 | 3 | 3 |
| Multiple vessel | 86 | 88 | 8 | 10 | — | — | 48 | 51 |
| ACC/AHA class | ||||||||
| A | 5 | 5 | 44 | 43 | 29 | 27 | 39 (A + B1) | 32 (A + B1) |
| B1 | 26 | 36 | 40 | 30 | 31 | 33 | ||
| B2 | 47 | 39 | 14 | 27 | 35 | 37 | 61 (B2 + C) | 68 (B2 + C) |
| C | 23 | 20 | 2 | 0 | 5 | 3 | ||
| Multilesion intervention | 30 | 16 | — | — | — | — | — | — |
Table 2 lists the study design and clinical outcomes. A loading dose (ranging from 4 to 10 mg) of sirolimus was used in 3 of the trials. Total duration of sirolimus use was 30 days in 2 studies and 14 days in the other 2 studies. Aspirin was used in all groups after coronary intervention and a thienopyridine was given up to 1 month in the BMS group and up to 1 year after DES. The duration of follow-up ranged from 7 months to 5 years. Major adverse cardiac events including death, cardiac death, and myocardial infarction were similar in both groups across these studies ( Table 2 ). The incidence of target vessel revascularization and TLR was significantly lesser in oral sirolimus group as reported in studies 1 and 2. There was a trend toward lower target vessel revascularization and TLR rates in studies 3 and 4 but did not reach statistical significance. Target vessel failure was reported in studies 1 and 3, and although there was no statistical difference, it did show a trend in favor of oral sirolimus. Cost analysis in study 4 also showed a significant cost saving using BMS and oral sirolimus compared with DES for initial procedure.
| Characteristic | Rodriguez et al | Stojkovic et al | Cernigliaro et al | Rodriguez et al | ||||
|---|---|---|---|---|---|---|---|---|
| Oral Sirolimus + BMS Group | Control Group | Oral Sirolimus + BMS Group | Control Group | Oral sirolimus + BMS Group | Placebo + BMS Group | Oral Sirolimus + BMS Group | DES Group | |
| Clinical follow-up duration (mo) | 31 | 6.8 + 1.5 | 60 | 36 | ||||
| Sirolimus dose | 6 mg loading dose followed by 3 mg/day for 14 days + diltiazem 180 mg/day | 2 mg/day for 30 days | 4 mg loading dose followed by 2 mg/day for 30 days | 10 mg loading dose day before intervention, followed by 3 mg/day for 14 days + diltiazem 180 mg/day | ||||
| Aspirin (mg/day) | 325 | 100 | 100 | 325 | ||||
| Clopidogrel (Bristol-Myers Squibb, New York) | 300 mg loading dose and 75 mg/day for 1 month | 75 mg/day 5 days before procedure and 1 month thereafter | 600 mg loading dose and 75 mg/day for 1 month | 300 mg loading dose and 75 mg/day/month | ||||
| Ticlopidine | Not used | Not used | 1,000 mg loading dose and 500 mg/day for 1 month, in 5% of patients | Not used | ||||
| Statin (%) | 100 | — | 100 | 100 | 100 | |||
| Death (%) | 4 | 4 | 0 | 0 | 9 | 6 | 5 | 11 |
| Cardiac death (%) | — | — | — | — | 6 | 4 | 2 | 5 |
| Myocardial infarction (%) | 4 | 2 | 0 | 0 | 0 | 2 | 6 | 11 |
| Stroke (%) | 2 | 2 | 0 | 0 | — | 0 | 1 | |
| TLR (%) | 85 | 37 | 7 | 23 | 26 | 44 | 10 | 14 |
| Target vessel revascularization (%) | 8 | 38 | 7 | 23 | 28 | 44 | 15 | 18 |
| Target vessel failure (%) | 18 | 44 | — | — | 33 | 50 | 25 | 32 |
| Any adverse cardiac events (%) | 20 | 44 | 7 | 23 | 31 | 50 | 11 | 20 |
| Overall cost (+taxes) per patient at 1 and 3 yrs ($) | — | — | — | — | — | — | 5,365.5 ± 876.0, 6,998.1 ± 3,385.6 | 6,921.5 ± 2,251.4, 11,201.5 ± 6,422.6 |
Table 3 lists the angiographic data. Pre and postintervention lesion characteristics showed no differences between the comparison groups. Routine follow-up coronary angiograms were reported in 3 of the 4 studies. In-stent and in-segment minimal luminal diameter, diameter stenosis, late loss, and ISR were reported variably across the studies. The trend however was in favor of oral sirolimus. In-stent minimal luminal diameter, in-segment minimal luminal diameter, and in-stent and in-segment late loss at follow-up favored the oral sirolimus group. Correspondingly, in-stent and in-segment diameter stenosis was significantly better in oral sirolimus group.
| Characteristic | Rodriguez et al | Stojkovic et al | Cernigliaro et al | Rodriguez et al | ||||
|---|---|---|---|---|---|---|---|---|
| Oral Sirolimus + BMS Group | Control Group | Oral Sirolimus + BMS Group | Control Group | Oral Sirolimus + BMS Group | Control Group | Oral Sirolimus + BMS | DES | |
| Before intervention | ||||||||
| Reference diameter (mm) | 2.96 ± 0.64 | 2.91 ± 0.41 | 2.58 ± 0.47 | 2.60 ± 0.4 | 2.92 ± 0.68 | 2.93 ± 0.76 | 2.8 ± 0.5 | 2.8 ± 0.4 |
| Minimal luminal diameter (mm) | 1.03 ± 0.43 | 0.98 ± 0.48 | 0.83 ± 0.32 | 0.86 ± 0.34 | 1.18 ± 0.63 | 1.21 ± 0.74 | — | — |
| Diameter stenosis (%) | 66.5 ± 12.2 | 65.68 ± 14.66 | 67.6 ± 11.18 | 67.1 ± 11.7 | 60.1 ± 16.4 | 60.6 ± 18.3 | — | — |
| After intervention | ||||||||
| Reference diameter (mm) | 3.02 ± 0.44 | 2.94 ± 0.36 | 2.67 ± 0.43 | 2.73 ± 0.36 | 3.13 ± 0.66 | 3.07 ± 0.7 | — | — |
| In-stent minimal luminal diameter (mm) | 2.99 ± 0.48 | 2.92 ± 0.50 | 2.44 ± 0.37 | 2.56 ± 0.29 | — | — | — | — |
| In-segment minimal luminal diameter (mm) | 2.7 ± 0.37 | 2.62 ± 0.38 | 2.22 ± 0.45 | 2.25 ± 0.36 | 2.76 ± 0.68 | 2.71 ± 0.65 | — | — |
| In-stent diameter stenosis (%) | 4.8 ± 10.7 | 4.7 ± 10.6 | 7.8 ± 9 | 5.7 ± 7.6 | — | — | — | — |
| In-segment diameter stenosis (%) | 11.7 ± 5.6 | 11.3 ± 7.27 | 16.2 ± 8.8 | 16.2 ± 8.6 | 13.0 ± 8.3 | 11.1 ± 7.6 | — | — |
| In-segment acute gain (mm) | 1.68 ± 0.43 | 1.76 ± 0.49 | 1.37 ± 0.5 | 1.46 ± 0.43 | 1.58 ± 0.58 | 1.50 ± 0.65 | — | — |
| Follow-up angiogram | 285 ± 54 days | 204 days | 6 months | Only on the basis of clinical indication | ||||
| Reference diameter (mm) | 2.95 ± 0.53 | 2.87 ± 0.45 | 2.67 ± 0.35 | 2.57 ± 0.43 | 2.65 ± 0.71 | 2.61 ± 0.74 | — | — |
| In-stent minimal luminal diameter (mm) | 2.26 ± 0.55 | 1.51 ± 0.65 | 2.19 ± 10.53 | 1.57 ± 0.7 | — | — | — | — |
| In-segment minimal luminal diameter (mm) | 2.04 ± 0.70 | 1.47 ± 0.76 | 1.99 ± 0.49 | 1.46 ± 0.65 | 1.97 ± 0.72 | 1.64 ± 0.85 | — | — |
| In-stent diameter stenosis (%) | 19.6 ± 17.7 | 54 ± 20.8 | 17.7 ± 17.2 | 39.2 ± 25.7 | — | — | — | — |
| In-segment diameter stenosis (%) | 32.7 ± 20.15 | 55.7 ± 25.01 | 25.2 ± 15.6 | 41.7 ± 24.2 | — | — | — | — |
| In-stent late loss (mm) | 0.73 ± 0.40 | 1.41 ± 0.67 | 0.32 ± 0.44 | 1.01 ± 0.69 | — | — | — | — |
| In-segment late loss (mm) | 0.66 ± 0.59 | 1.13 ± 0.72 | 0.29 ± 0.39 | 0.86 ± 0.64 | 0.79 ± 0.68 | 1.07 ± 0.55 | — | — |
| Lesion length (mm) | 13.35 ± 6.33 | 12.79 ± 4.28 | 9.48 ± 3.68 | 8.95 ± 2.81 | 13.90 ± 6.0 | 13.84 ± 5.9 | 13.8 ± 5.5 | 14.4 ± 5.9 |
| Stent length (mm) | 15.7 ± 2.62 | 16 ± 2.78 | 17.37 ± 2.44 | 17.61 ± 2.2 | 15.2 ± 7.6 | 14.5 ± 5.38 | 19.1 ± 4.4 | 21.4 ± 5.2 |
| In-segment net gain (mm) | 0.97 ± 0.62 | 0.49 ± 0.48 | 1.16 ± 0.59 | 0.60 ± 0.64 | — | — | — | — |
| In-segment restenosis (%) | 12 | 45 | 11 | 51 | — | — | — | — |
| Per vessel (%) | 12 | 43 | 10 | 51 | — | — | ||
| ISR (%) | 12 | 36 | 9 | 49 | — | — | — | — |
| Per vessel (%) | 12 | 35 | 8 | 49 | — | — | — | — |
| Per lesion (%) | — | — | — | — | 14 | 32 | — | — |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


