The presence of functional mitral regurgitation (MR) is considered a significant risk factor for poor clinical prognosis in patients with idiopathic dilated cardiomyopathy (IDC). The objectives of this study were to test the hypothesis that not only global but also local left ventricular (LV) remodeling, including the position of the papillary muscles, may contribute to the development of MR in patients with IDC and wide QRS durations and can be reversed with cardiac resynchronization therapy (CRT). Eighty-four subjects were studied, 44 patients with IDC who underwent CRT and 40 age- and gender-matched controls. The position of the posteromedial papillary muscle was similar in the 2 groups, whereas the position of the anterolateral papillary muscle in patients with IDC was displaced more posteriorly than in controls. Multivariate analysis revealed that reduction in coaptation height (β = 0.44, p <0.001) and LV dyssynchrony by speckle-tracking radial strain (β = 0.303, p <0.01) were independent determinants of reduction in MR 5 ± 2 days after CRT; in contrast, restoration of the position of the posteriorly displaced anterolateral papillary muscle (β = 0.50, p <0.001) and the increase in sphericity index (β = 0.440, p <0.001) were identified as independent determinants of reduction in MR 6 ± 1 months after CRT. In conclusion, asymmetric local LV remodeling was observed at baseline, and asymmetric local LV reverse remodeling was observed at long-term follow-up after CRT in patients with IDC. Furthermore, different parameters contribute to the reduction in MR observed at short- and long-term follow-up after CRT.
Cardiac resynchronization therapy (CRT) is an established therapeutic option for symptomatic heart failure (HF) patients with severely depressed left ventricular (LV) systolic function and wide QRS durations. Previous randomized trials and single-center studies have demonstrated that CRT leads to reductions in functional mitral regurgitation (MR) in the short-term and long-term phases after CRT. However, the underlying mechanisms of short- and long-term reductions in MR after CRT have not been fully elucidated. Accordingly, the objectives of this study were to test the hypothesis that not only global but also local LV morphologic alteration may contribute to the development of MR in patients with severely remodeled hearts and wide QRS durations and can be reversed with CRT.
Methods
We studied consecutive HF patients with idiopathic dilated cardiomyopathy (IDC) and moderate or severe MR for whom complete data sets consisting of baseline and short- and long-term follow-up echocardiographic data were available. The diagnosis of IDC was supported by coronary angiography, and we excluded patients with (1) coronary artery disease, defined as ≥1 stenoses >75% of a major epicardial vessel or a history of myocardial infarction; (2) other known causes of cardiomyopathy; and (3) morphologic abnormalities of the mitral apparatus, such as mitral valve prolapse or a restricted leaflet due to degenerative calcification. The control group consisted of 40 age- and gender-matched healthy volunteers with no histories of cardiovascular disease and with completely normal electrocardiographic findings and 2-dimensional and Doppler echocardiographic results. Written informed consent was obtained from all subjects.
Echocardiographic studies were performed using a commercially available echocardiographic system (Aplio XG; Toshiba Medical Systems, Tochigi, Japan). All patients were studied at baseline, short-term follow-up at 5 ± 2 days, and long-term follow-up at 6 ± 1 months after CRT. Digital routine grayscale 2-dimensional cine loops from 3 consecutive beats were obtained from standard apical views (4 chamber, 2 chamber, and long axis) and the mid-LV short-axis view. Sector width was optimized to allow complete myocardial visualization while maximizing the frame rate. Color Doppler echocardiography was performed with an aliasing velocity of 65 cm/s. Finally, digital data were transferred to dedicated software (EchoAgent; Toshiba Medical Systems) for subsequent off-line analysis.
LV dyssynchrony was assessed from the mid-LV short-axis view using speckle-tracking radial strain, as previously described in detail. A circular region of interest traced the endocardium at end-systole using a point-and-click approach. A second larger concentric circle was then automatically generated and manually adjusted near the epicardium or manually traced. The mid-LV image was divided into 6 standard segments, and time-strain curves were generated from each segment. LV dyssynchrony was defined as the time difference between anteroseptal and posterior wall segmental peak strain. Similarly, the time difference of peak strain between the anterolateral papillary muscle (APM) and posteromedial papillary muscle (PPM) was defined as inter–papillary muscle dyssynchrony.
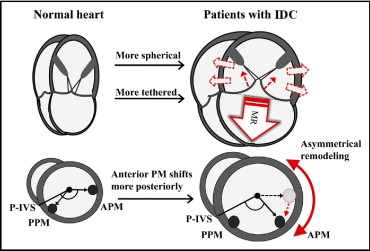
LV end-diastolic volume, end-systolic volume (ESV), and ejection fraction were assessed using the biplane Simpson’s rule using manual tracing of digital images. A response to CRT was defined as reverse remodeling detected by a relative decrease of ≥15% in ESV at long-term follow-up. To evaluate LV spherical remodeling, the sphericity index was calculated as the ratio of the major to the minor axis of LV end-systolic dimensions in the apical 4-chamber view ( Figure 1 ) . LV volumes and sphericity index were considered the parameters of global LV remodeling.

To clarify the morphologic characteristics of local LV remodeling, we evaluated the degree of circumferential displacement of the papillary muscles from the mid-LV short-axis view at end-systole. The posterior ventricular sulcus was used as an anatomic landmark to quantify the circumferential displacement of the papillary muscles. Angle α was defined as the angle between the posterior ventricular sulcus, contractile center, and the insertion of the PPM and angle β as the angle between the posterior ventricular sulcus, contractile center, and the insertion of the APM ( Figure 1 ). Angles α and β were considered the parameters of local LV remodeling.
The quantification of MR was performed in a parasternal long-axis view or in an apical long-axis view by measuring the width of the vena contracta. MR was also calculated planimetrically as the percentage of the MR jet area in relation to the left atrial area and averaged in the apical 4-chamber, 2-chamber, and long-axis views. Color flow Doppler settings were kept constant during the entire study. The severity of MR was graded as mild (vena contracta <3.0 mm), moderate (vena contracta 3.0 to 6.9 mm), or severe (vena contracta ≥7.0 mm) on the basis of the recommendations of the American Society of Echocardiography. Mitral annular diameter was measured at end-diastole in the parasternal long-axis view from the base of the aortic cusp at the posterior aortic root to the hinge of the posterior mitral leaflet ( Figure 1 ). Mitral annular area was obtained by determining the annular dimensions in the apical 4- and 2-chamber views, followed by calculation of mitral annular area as d1 × d2 × π/4. Coaptation height was determined as the displacement of mitral coaptation toward the LV apex by measuring the distance between leaflet coaptation and the mitral annular plane in the apical 4-chamber view ( Figure 1 ). The mitral annular diameter, mitral annular area, and coaptation height were considered the mitral deformation parameters.
Continuous variables are expressed as mean ± SD. Group comparisons between before and after CRT were performed using paired Student’s t tests and group comparisons between responders and nonresponders using unpaired Student’s t tests. Proportional differences were evaluated using Fisher’s exact tests or chi-square tests as appropriate. Univariate linear regression analysis was initially used to assess the relation between the reduction in MR and the changes in global and local LV remodeling, mitral deformation, and dyssynchrony parameters. For analysis of the independent determinants of the reduction in MR, multiple linear regression analysis on the basis of stepwise selection was performed. The entry criterion for the multivariate linear regression model was a univariate p value <0.05. For all tests, p values <0.05 were considered statistically significant. All analyses were performed using SPSS version 13.0 (SPSS, Inc., Chicago, Illinois).
Results
The study group consisted of 53 consecutive HF patients with IDC referred for CRT. Three patients (6%) were excluded from all subsequent analyses because of inadequate image quality for echocardiographic analysis, and we also excluded 6 patients (11%) with mild MR. The final patient study group thus consisted of 44 patients with IDC with moderate or severe MR ( Table 1 ). As expected, patients with IDC exhibited significant global LV remodeling compared to normal controls (end-diastolic volume 210 ± 70 vs 80 ± 19 ml, ESV 151 ± 65 vs 30 ± 8 ml, ejection fraction 28 ± 5% vs 63 ± 8%, all p values <0.001). The left ventricles of patients with IDC were also more spherical than those in normal controls (sphericity index 1.54 ± 0.17 vs 1.94 ± 0.25, p <0.001). Furthermore, the left ventricles in patients with IDC exhibited significant local LV remodeling. Of interest, the circumferential position of the PPM in patients with IDC was similar to that in normal controls (angle α 31 ± 11° vs 30 ± 9°), whereas the circumferential position of the APM in patients with IDC was displaced more posteriorly than that in normal controls (angle β 147 ± 10° vs 173 ± 14°, p <0.001). This indicated that asymmetric local remodeling had occurred in the failing hearts ( Figure 2 ) .
| Variable | Controls | Patients with IDC | p Value |
|---|---|---|---|
| (n = 40) | (n = 44) | ||
| Age (years) | 70 ± 9 | 70 ± 7 | NS |
| Men/women | 28/12 | 31/13 | NS |
| Systolic blood pressure (mm Hg) | 120 ± 14 | 108 ± 15 | <0.001 |
| Diastolic blood pressure (mm Hg) | 72 ± 6 | 61 ± 6 | <0.001 |
| Heart rate (beats/min) | 69 ± 6 | 67 ± 8 | <0.05 |
| Ejection fraction (%) | 63 ± 4 | 28 ± 5 | <0.001 |
| Sphericity index | 1.9 ± 0.3 | 1.5 ± 0.2 | <0.001 |
| Angle α (°) | 30.3 ± 9.3 | 30.9 ± 11.0 | NS |
| Angle β (°) | 173 ± 14 | 147 ± 10 | <0.001 |
| Coaptation height (mm) | 3.1 ± 1.5 | 7.3 ± 2.1 | <0.001 |
| Mitral annular dimension (mm) | 30.5 ± 3.4 | 37.4 ± 6.1 | <0.001 |
| Mitral annular area (cm 2 ) | 5.7 ± 1.6 | 10.2 ± 2.0 | <0.001 |
| Vena contracta width (mm) | |||
| Moderate (3.0–6.9) | 0 | 22 (61%) | <0.001 |
| Severe (≥7.0) | 0 | 22 (39%) | <0.001 |
| New York Heart Association functional class | |||
| III | 0 | 36 (82%) | <0.001 |
| IV | 0 | 8 (18%) | <0.001 |
Responders and nonresponders were of similar ages and showed a similar gender distribution and QRS durations ( Table 2 ). Moreover, the 2 groups showed similar global LV remodeling, including LV volumes, ejection fractions, and sphericity indexes, but responders were more likely to have greater LV dyssynchrony (304 ± 87 vs 166 ± 122 ms, p <0.001) and greater inter–papillary muscle dyssynchrony (115 ± 46 vs 69 ± 41 ms, p <0.001) compared to nonresponders at baseline. Responders showed significant global LV reverse remodeling at long-term follow-up ( Table 3 ), as evidenced by decreases in end-diastolic volume and ESV and increases in sphericity index and ejection fraction, but not at short-term follow-up. For nonresponders, in contrast, all parameters of global LV reverse remodeling were not changed between baseline and short- and long-term follow-up.
| Variable | Responders | Nonresponders |
|---|---|---|
| (n = 31) | (n = 13) | |
| Age (years) | 70 ± 10 | 70 ± 7 |
| Men/women | 22/9 | 7/6 |
| Systolic blood pressure (mm Hg) | 109 ± 15 | 103 ± 11 |
| Diastolic blood pressure (mm Hg) | 61 ± 7 | 61 ± 6 |
| Heart rate (beats/min) | 68 ± 8 | 66 ± 7 |
| QRS duration (ms) | 161 ± 20 | 160 ± 19 |
| End-diastolic volume (ml) | 210 ± 69 | 209 ± 73 |
| ESV (ml) | 153 ± 61 | 148 ± 75 |
| Ejection fraction (%) | 28 ± 5 | 30 ± 6 |
| Sphericity index | 1.6 ± 0.2 | 1.5 ± 0.2 |
| Angle α (°) | 31 ± 9 | 30 ± 13 |
| Angle β (°) | 146 ± 10 | 149 ± 8 |
| Coaptation height (mm) | 7.3 ± 1.7 | 7.2 ± 2.9 |
| Mitral annular dimension (mm) | 39 ± 4 | 41 ± 4 |
| Mitral annular area (cm 2 ) | 10.2 ± 2.1 | 10.4 ± 1.7 |
| Vena contracta width (mm) | 6.7 ± 1.9 | 6.8 ± 1.5 |
| MR jet area/left atrial area (%) | 38 ± 13 | 38 ± 9 |
| Left ventricular dyssynchrony (ms) | 304 ± 87 ⁎ | 166 ± 122 ⁎ |
| Inter–papillary muscle dyssynchrony (ms) | 115 ± 46 ⁎ | 69 ± 41 ⁎ |
| New York Heart Association functional class | ||
| III | 25 (81%) | 11 (85%) |
| IV | 6 (19%) | 2 (15%) |
| Medications | ||
| Loop diuretics | 28 (90%) | 11 (85%) |
| Spironolactone | 21 (68%) | 7 (54%) |
| β blockers | 28 (90%) | 11 (85%) |
| Angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers | 28 (90%) | 10 (77%) |
| Variable | Responders (n = 31) | Nonresponders (n = 13) | ||||
|---|---|---|---|---|---|---|
| Baseline | Short-Term Follow-Up | Long-Term Follow-Up | Baseline | Short-Term Follow-Up | Long-Term Follow-Up | |
| Hemodynamics | ||||||
| Systolic blood pressure (mm Hg) | 109 ± 15 | 109 ± 13 | 107 ± 15 | 103 ± 11 | 102 ± 12 | 96 ± 16 |
| Diastolic blood pressure (mm Hg) | 61 ± 7 | 61 ± 7 | 63 ± 8 | 61 ± 6 | 59 ± 6 | 58 ± 12 |
| Heart rate (beats/min) | 68 ± 8 | 67 ± 7 | 69 ± 8 | 66 ± 7 | 66 ± 5 | 71 ± 9 |
| Global LV remodeling | ||||||
| End-diastolic volume (ml) | 210 ± 69 | 207 ± 80 | 117 ± 51 ⁎ † | 209 ± 73 | 210 ± 91 | 207 ± 73 |
| ESV (ml) | 153 ± 61 | 149 ± 72 | 75 ± 39 ⁎ † | 148 ± 88 | 148 ± 63 | 149 ± 82 |
| Ejection fraction (%) | 28 ± 5 | 28 ± 6 | 36 ± 8 ⁎ † | 30 ± 6 | 30 ± 5 | 28 ± 4 |
| Sphericity index | 1.6 ± 0.2 | 1.6 ± 0.2 | 1.8 ± 0.2 ⁎ † | 1.5 ± 0.2 | 1.5 ± 0.2 | 1.5 ± 0.2 |
| Local LV remodeling | ||||||
| Angle α (°) | 31 ± 9 | 30 ± 8 | 31 ± 12 | 30 ± 13 | 30 ± 11 | 32 ± 15 |
| Angle β (°) | 146 ± 10 | 146 ± 10 | 164 ± 10 ⁎ † | 149 ± 8 | 149 ± 8 | 153 ± 8 |
| Mitral deformation indexes | ||||||
| Coaptation height (mm) | 7.3 ± 1.7 | 5.8 ± 1.6 ⁎ | 4.4 ± 2.2 ⁎ † | 7.2 ± 2.9 | 6.9 ± 1.9 | 6.9 ± 1.8 |
| Mitral annular dimension (mm) | 39 ± 4 | 39 ± 4 | 38 ± 5 | 40 ± 4 | 41 ± 4 | 42 ± 5 |
| Mitral annular area (cm 2 ) | 10.2 ± 2.1 | 10.0 ± 2.1 | 9.8 ± 2.0 | 10.4 ± 1.7 | 10.8 ± 1.7 | 10.9 ± 1.8 |
| MR | ||||||
| Vena contracta width (mm) | 6.7 ± 1.9 | 4.5 ± 2.1 ⁎ | 2.1 ± 1.9 ⁎ † | 6.8 ± 1.5 | 6.6 ± 1.3 | 6.4 ± 1.3 |
| MR jet area/left atrial area (%) | 38 ± 13 | 25 ± 12 ⁎ | 12 ± 10 ⁎ † | 38 ± 9 | 36 ± 8 | 36 ± 9 |
| Dyssynchrony | ||||||
| LV dyssynchrony (ms) | 304 ± 87 | 123 ± 115 ⁎ | 83 ± 98 ⁎ ‡ | 166 ± 122 | 167 ± 76 | 116 ± 64 |
| Inter–papillary muscle dyssynchrony (ms) | 115 ± 46 | 69 ± 38 ⁎ | 53 ± 35 | 69 ± 41 | 76 ± 52 | 79 ± 47 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


