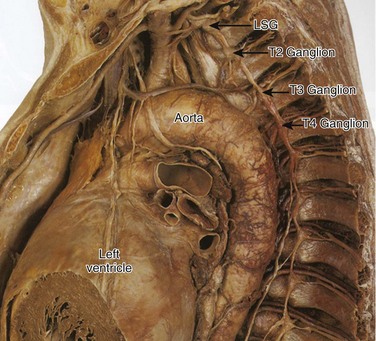
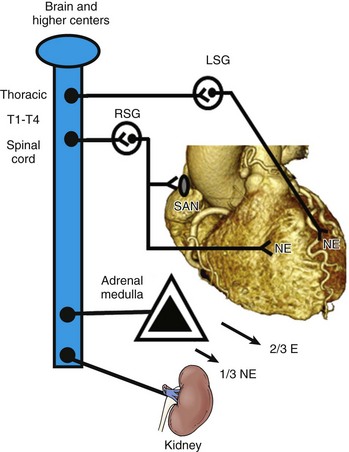
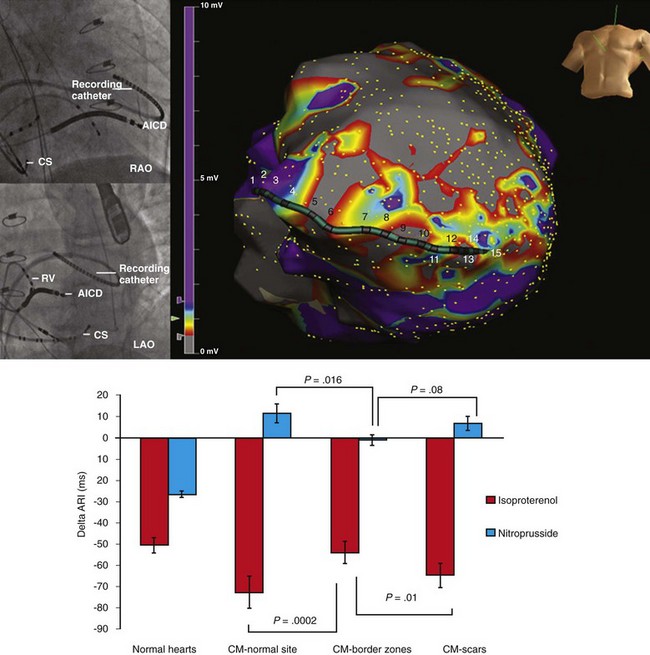
41 Anatomy of Cardiac Sympathetic Innervation Sympathetic Modulation of Myocardial Excitability Neural Remodeling in the Presence of Myocardial Pathology Proarrhythmic Effects of Sympathetic Stimulation Effect of Neuraxial Modulation in Reducing Risk of Ventricular Arrhythmias Surgical Sympathetic Denervation in Humans The autonomic nervous system plays a significant role in the genesis and maintenance of ventricular arrhythmias, and powerfully modulates the underlying substrate in a dynamic manner.1–3 Blockade of the sympathetic nervous system, whether through medications or neuraxial modulation, has been associated with a reduction in the risk of sudden cardiac death and the burden of ventricular arrhythmias. In this chapter, the anatomy of the cardiac sympathetic nervous system and pathological changes associated with neural remodeling in the setting of scar and cardiomyopathy will be reviewed. Subsequently, the role of sympathetic activation in promoting arrhythmias, and the effects of neuraxial modulation in reducing the burden of these arrhythmias will be discussed. Finally, cardiac denervation in humans, including feasibility, surgical techniques, and complications, will be considered. The preganglionic sympathetic neurons that innervate the heart reside in the intermediate zone of the thoracic spinal cord.4 These preganglionic sympathetic nerves pass through the white rami communicantes, enter the sympathetic trunk, and terminate in the cervicothoracic ganglion as well as in the T2-T4 ganglia. Of note, preganglionic neurons may synapse on neurons within the ganglia at the same thoracic level or may travel within the sympathetic chain and synapse on neurons of ganglia at other spinal levels. The preganglionic neurotransmitter within the ganglia is acetylcholine. The right and left cervicothoracic ganglia, which are often a product of the fusion of the C8 and T1 ganglia, are called the left stellate (LSG) and right stellate (RSG) ganglia. LSG and RSG, along with the ganglia of T2-T4, give rise to postganglionic axons that target organs including the heart, the esophagus, the trachea, and the head and neck (Figure 41-1). Postganglionic nerve fibers from these ganglia then join the branches of the vagus nerve to form the left and right cardiac (cardiopulmonary) nerves, destined for the heart.5,6 These nerves subsequently form a network of neurons on the epicardium, called the cardiac plexus. The cardiac plexus, which is divided into the superficial (ventral) and deep (dorsal) cardiac plexus, gives rise to sympathetic nerve branches that run along the coronary arteries on the epicardium and penetrate the myocardium with the vasculature. Thus, the nerves are located primarily around blood vessels and between myocytes oriented along their long axis. Further, a gradient in sympathetic innervation is thought to exist from base to apex, as well as from epicardium to endocardium.7,8 Figure 41-1 The sympathetic chain and in particular the left stellate ganglion (LSG) and the ganglia of thoracic spinal 1-4 (T1-T4) are located in a paravertebral position behind the parietal pleura. (Reproduced with permission from Abrahams PH, Spratt JD, Boon J: McMinn’s Clinical Atlas of Human Anatomy, 6th ed, St Louis, 2007, Mosby.) Data collected over the past four decades indicate that extensive neural processing occurs in the cardiac plexus, otherwise known as the intrinsic cardiac nervous system. This plexus contains afferent neurons, interconnecting interneurons, and sympathetic and parasympathetic efferent postganglionic neurons.9 Furthermore, it is composed of seven ganglionic subplexi, containing more than 800 epicardial ganglia. Each subplexus or group of subplexi innervates different chambers of the heart. One subplexus innervates the right ventricle, three innervate the left ventricle, and the rest innervate the atria. The highest density of epicardial ganglia, approximately 50%, exists near the hilum of the heart, especially on the dorsal and dorsolateral surfaces of the left atrium. The number of neurons in these ganglia decreases with age, from approximately 94,000 neurons in neonates and children to 43,000 intrinsic neurons in the plexus of adults.10 A complex feedback regulatory system allows the cardiac nervous system to modulate sympathetic and parasympathetic input to the heart. Cardiac nerves can be demonstrated by labeling nerve-specific markers such as S100 protein, neurofilament, and synaptophysin using immunohistochemistry techniques,5,6 and myelinated fibers can be identified by myelin markers, such as myelin basic protein. Sympathetic nerves can be identified by immunolabeling tyrosine hydroxylase. Tyrosine hydroxylase and myelin basic protein staining have confirmed the presence of myelinated sympathetic nerves on all surfaces of the epicardium and endocardium.11 The major neurotransmitter mediating sympathetic response in the heart is norepinephrine. Epinephrine release from intracardiac neural endings is negligible.12 Along the length of terminal axons are a series of localized swellings known as varicosities. Most of the norepinephrine storage vesicles in a terminal axon are concentrated in these varicosities, which act as specialized sites of norepinephrine storage and release.13 The overall effect of norepinephrine release through multiple signaling pathways is shortening of the ventricular action potential duration (APD) and the refractory period.14 Most norepinephrine undergoes reuptake into nerve terminals by the presynaptic norepinephrine transporter. A small fraction diffuses into the vascular space, where it can be measured in coronary sinus blood. Norepinephrine spillover (both interstitial and in the coronary sinus) can be used to infer that sympathetic outflow to the heart can also be assessed in humans.15 Sympathetic stimulation, predominantly mediated by postsynaptic myocardial β-adrenergic receptors, has important effects on chronotropy, dromotropy, lusitropy, and inotropy. Discharge of the sinoatrial (SA) node and atrioventricular (AV) nodal conduction are augmented, increasing chronotropy. In the atria and ventricles, contractility and relaxation are enhanced. Both β1 and β2 subtypes are present at a ratio of approximately 5 : 1 in the healthy human heart.16–18 Alpha adrenoreceptors are mainly present in the vascular wall, but are also found in ventricular myocardium, where they account for approximately 15% of cardiac adrenergic receptors.18 Cardiac sympathetic activation is complex and is mediated through multiple organs at multiple levels. The brain (higher centers), brain stem, spinal cord, sympathetic ganglia, adrenal medulla, and renal nerves can increase the net sympathetic output to the heart, dynamically modulating cardiac function (Figure 41-2). Figure 41-2 Multiple organs mediate sympathetic outflow to the normal heart including the brain, the spinal cord, the sympathetic chain (left stellate ganglion [LSG], right stellate ganglion [RSG], and ganglia of T1-T4), the adrenal medulla by secreting catecholamines, and the renal nerves. E, Epinephrine; LSG, left stellate ganglion; NE, norepinephrine; RSG, right stellate ganglion; SAN, sinoatrial node; T1-T4, thoracic spinal level 1 through 4. Myocardial infarction causes death of sympathetic fibers within the scar and loss of efferent sympathetic innervation at noninfarcted apical sites.19 Norepinephrine depletion in the scar is accompanied by increased production in noninfarcted basal areas.20 In response to LSG stimulation, viable myocardial sites apical to an infarct show evidence of heterogeneity in innervation; certain viable sites shorten their repolarization, while others show no response. All denervated areas show denervation super-sensitivity, defined as an exaggerated response to norepinephrine infusion.21 The cellular mechanisms for this response do not involve differences in the β-adrenergic receptor or the α-subunit of stimulatory G-protein density.22,23 In patients with recurrent ventricular arrhythmias undergoing ventricular tachycardia (VT) ablation procedures, activation recovery interval (ARI) measurements show a reduced response to indirect sympathetic stimulation via nitroprusside in dense scar and in the viable peri-infarct myocardium, suggesting denervation.24 These sites demonstrate an exaggerated response to isoproterenol infusion, suggesting the presence of denervation super-sensitivity in humans (Figure 41-3). Further, the response to sympathetic stimulation is extremely heterogeneous, with a greater than 2-fold increase in dispersion in ARI with nitroprusside infusion.24 Figure 41-3 A recording multi-electrode catheter on fluoroscopy (left upper panels) and electroanatomical map (right upper panel) in this patient with ischemic cardiomyopathy (ICM) and a large anteroapical scar are used to record unipolar electrograms from scar, border zone, and viable myocardium. On the electroanatomical map, the purple areas represent viable tissue (normal voltage) and gray represents dense scar. All other colors represent border zones (0.5 mV < voltage < 1.5 mV). The delta activation recovery interval (ARI; change in ARI from baseline) within the cardiomyopathic and normal hearts is shown in the lower panel. Note that in response to isoproterenol, the delta ARI is greatest in the CM-normal site (viable myocardium) and scar regions of the cardiomyopathic heart. The border zones within each patient are the least responsive to isoproterenol. On the other hand, in response to nitroprusside, the scar and the CM-NL tissue appear to be the least responsive, even paradoxically increasing their ARI in comparison with border-zone regions. Therefore, it appears that the most denervated regions have the greatest response to catecholamines, consistent with denervation super-sensitivity. After studies showed acute denervation, evidence of nerve sprouting and heterogeneous hyperinnervation was observed in chronic MI and heart failure models. Peripheral nerve injury resulting in Wallerian degeneration, which leads to nerve growth factor (NGF), triggered regeneration via nerve sprouting.25,26 Along with denervation, MIBG studies have shown localized re-innervation in injured myocardium in both ischemic and nonischemic cardiomyopathy patients.27,28 In human hearts with cardiomyopathy, local increases in sympathetic nerves in a “swarm-like” pattern in the periphery of necrotic tissues and in perivascular regions have been observed, and these changes were more prominent in patients with a history of ventricular arrhythmias.29–31 These border zones of infarcts have also been shown to be frequent sites of origin of inducible ventricular tachycardia (VT)/ventricular fibrillation (VF).32,33 Furthermore, nerve sprouting can occur in a noninfarct setting such as stem cell transplantation,34 in radiofrequency ablation,35 and in rapid pacing–induced heart failure in dogs36 and in hypercholesterolemic rabbits, where it has been shown to cause QTc dispersion, increased heterogeneity of repolarization, and significantly increased episodes of VF, both spontaneous and induced.37 Furthermore, NGF infusion into the LSG promotes nerve sprouting in dogs with MI and complete AV block, and is associated with increased incidence of VF.38 Infusion of NGF into the RSG has not been shown to increase the risk of sudden cardiac death (SCD).39 Of note, NGF and GAP-43 levels are increased in the LSG of these dogs 3 days after MI, without a concomitant increase in mRNA levels, indicating possible retrograde transportation of these proteins to the LSG, which then triggers nerve sprouting at noninfarcted LV sites.40 Heart failure can cause trans-differentiation of cardiac sympathetic nerves. Cholinergic trans-differentiation of nerve sprouts by production of interleukin-6 cytokines through a gp-130 signaling pathway has been demonstrated in rodents,41,42 potentially further promoting heterogeneity in repolarization. However, the exact ramifications of this sympathetic rejuvenation and plasticity are yet unknown. Heart failure is also known to cause remodeling of cardiac ion channels, including increased L-type Ca (ICaL) density, decreased potassium current, decreased Ito density, and changes in Cl and Ca transporters and enzymes in the border zones surrounding the infarct.43–46 Thus, sympathetic stimulation could result in complex effects on the APD and restitution, which, along with increased ICaL density, can lead to intracellular Ca2+ overload–induced triggered activity, potentiating the risk of spontaneous ventricular arrhythmias. In addition to cardiac neural remodeling, electroanatomical remodeling of the LSG in the setting of MI and heart failure has been described. Increased nerve density along with increased mRNA levels of NGF and GAP-43 in the LSG of dogs with MI and nerve sprouting has been reported.40 Increased stellate ganglion nerve activity (SGNA) immediately after MI was associated with intramyocardial nerve sprouts, as well as increased neuronal size and synaptic density in the LSG and RSG.47 Although SCD was not observed in this study, Ogawa et al and Zhou et al had previously shown that sympathetic nerve discharges tend to precede ventricular arrhythmias in the same dog model of MI.48,49 Similarly, in human cadavers with evidence of cardiac scar, neuronal number in the LSG is increased as compared with cadavers without evidence of scar.50 Compared with cadavers with normal hearts, those with cardiomyopathy demonstrate increased neuronal size and synaptophysin density (Figure 41-4).51
Sympathetic Innervation, Denervation, and Cardiac Arrhythmias
Anatomy of Cardiac Sympathetic Innervation
Sympathetic Modulation of Myocardial Excitability
Neural Remodeling in the Presence of Myocardial Pathology
Cardiac Neural Remodeling and Denervation
Cardiac Neural Remodeling and Nerve Sprouting
Extracardiac Neural Remodeling
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Sympathetic Innervation, Denervation, and Cardiac Arrhythmias
