CHAPTER 79 Surgical Treatment of the Tricuspid Valve
SURGICAL ANATOMY
Tricuspid Valve Anulus
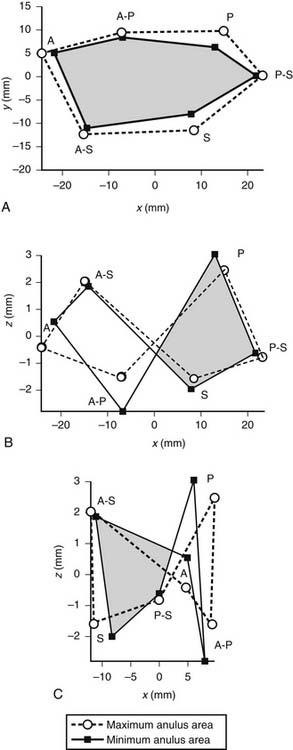
Whereas the base of the anterior and posterior leaflets is attached to the free wall of the right ventricle, the septal leaflet is inserted into the base of the interventricular septum. This line of leaflet attachment, known as the tricuspid valve anulus, is more a landmark than an actual fibrous ring. This absence of an encircling fibrotic structure explains the large changes in the tricuspid valve’s orifice during the cardiac cycle and its easy dilation in disease. The mobility and size of the tricuspid valve orifice are dependent on the transversely oriented myocardial fibers that surround the atrioventricular valves.1 Tsakiris and associates2 found in a canine model that the size of the tricuspid valve orifice changed continuously during the cardiac cycle. The orifice area contracted (from its maximal diastolic size) by 20% to 30%. Tei and associates3 confirmed these findings in humans by use of echocardiography. In our laboratory, we analyzed an ovine model for the changes in the normal tricuspid valve orifice during the cardiac cycle as detected by the changes in distance between ultrasound crystals placed around the line of insertion of the leaflets (Fig. 79-1).4,5 The tricuspid valve orifice area expands and contracts twice during the cardiac cycle. Orifice contraction begins during the isovolumic relaxation phase of the cardiac cycle and continues through the first half of diastole. Starting with the beginning of isovolumic contraction, a second contraction occurs during ejection, which reduces the tricuspid valve orifice to its minimum area. This contraction corresponds to closure of the valve completed at the end of isovolumic contraction. The reduction in orifice perimeter is not uniform. The segment of the anulus corresponding to the septal leaflet shortens by 12%, the anterior segment by 15%, and the posterior segment by 17%.4 The notion that the length of the septal portion of the anulus can be used to determine the size of an anuloplasty device should be revised.
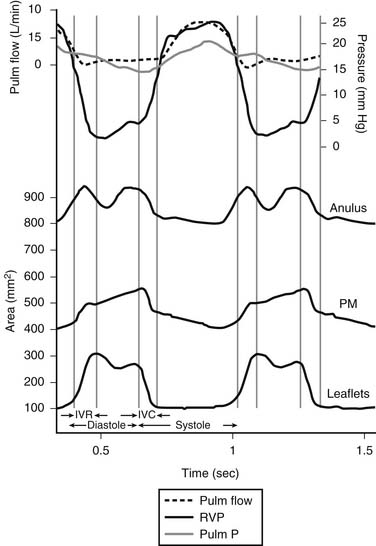
(Reproduced with permission from Jouan J, Pagel MR, Hiro ME, et al. Further information from a sonometric study of the normal tricuspid valve annulus in sheep: geometric changes during the cardiac cycle.5)
This narrowing of the tricuspid valve orifice is due not only to contraction of its perimeter but, more important, to changes in the shape of the anulus. During contraction, the orifice becomes more elliptical because of displacement of the anteroposterior commissure toward the septum and the bulging of the septum. As in the mitral valve, the “anulus” is not in a single plane. In fact, the tricuspid valve anulus is saddle shaped, with its horn or pommel corresponding to the area of the anteroseptal commissure and its cantle to the midpoint of the base of the posterior leaflet (Fig. 79-2). This saddle shape or hyperbolic paraboloid, well known to architects as an ideal design to reduce building tension, has been shown in the mitral valve to significantly reduce peak leaflet stress.6 Furthermore, the increase in the saddle shape during contraction has a folding, reducing effect on the normal tricuspid valve orifice. In cases of tricuspid regurgitation, besides an increase of tricuspid valve anulus area, there is an increase in planarity or flattening of the normal anulus, which induces leaflet tethering.7 Rigid structures, such as stented prostheses and rigid anuloplasty rings, destroy this configuration and (most likely) have a negative impact on the function of the right ventricle.
Leaflets
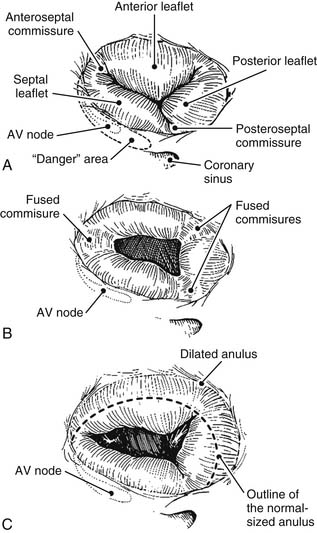
Although the number of tricuspid valve leaflets varies according to author,8 it is generally accepted that the tricuspid valve consists of three leaflets. These three leaflets are known as septal, anterior, and posterior, and they are separated by three clefts or commissures named anteroseptal, anteromedial, and posteroseptal (Fig.79-3A). These clefts do not reach the anulus but delineate small “commissural leaflets.” This is an important surgical point because in cases of fused commissures, their incision should not extend all the way to the anulus; doing so destroys the commissural leaflets. The largest leaflet is the anterior, followed by the posterior, with the septal being the smallest. The excursion of each leaflet (defined as the angle between leaflet free edge and the plane of the anulus) is different.5 The excursion of the septal leaflet is significantly smaller than that of the other leaflets. This normal, smaller septal excursion might explain the finding at surgery of a septal leaflet plastered against the septum. This finding, often seen in cases of functional regurgitation, is a sign of severity and a predictor of a poor result after repair.
Chordae Tendineae and Papillary Muscles
The leaflets are held down by marginal and basal chords that arise from three papillary muscle groups. The marginal chords are inserted into the leaflet’s free margin, and the basal chords are inserted into the ventricular surface of the leaflets. Elongation or rupture of the marginal chords results in leaflet prolapse. The tricuspid valve’s basal chords, although far less prominent than the mitral valve’s basal chords, probably play a similar role in maintaining valvular and ventricular geometry. In most tricuspid valve cases, three papillary muscle groups are found and can be identified as anterior, posterior, and septal. Whereas the anterior and posterior muscles are virtually always present, the septal muscle might be absent in 20% of patients. The anterior papillary muscle is the longest; it most often has a single head and sustains the largest number of chordae.9,10
Terminology
New surgical techniques always demand deeper knowledge of the anatomy of the region and, consequently, more precise terminology. A common set of anatomic definitions is needed to report precise preoperative and postoperative echocardiographic information important to both echocardiologist and surgeon. In an attempt to standardize all anatomic findings, we developed a detailed tricuspid valve terminology that parallels previously described mitral valve nomenclature.11 Although it is based on a surgical, atrial view of the valve, it encompasses all three elements of the tricuspid valve apparatus (i.e., leaflets, chords, and papillary muscles).10
Because the lesion does not often affect the whole leaflet or is limited to the chordae, each leaflet is divided into two areas identified by the papillary origin of the corresponding chords. For instance, the septal leaflet (S) is divided into two halves (S1 and S2), identified by the chords that originate from the anteroseptal papillary muscle1 or the chords that originate from the posteroseptal papillary muscle.2 The commissures (C) are identified by their papillary origin (1, 2, or 3) as C1, C2, and C3. All chords are identified by their papillary origin and leaflet insertion.
Anatomic Relationships
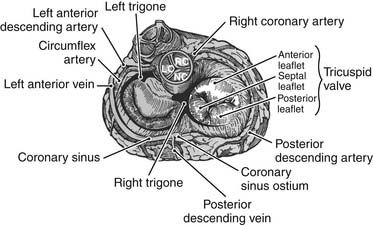
The area corresponding to the anteroseptal commissure is very close to the aortic valve’s noncoronary sinus of Valsalva (Fig.79-4). This fact has important surgical implications because placement of anchoring sutures for a tricuspid valve prosthetic device can be difficult at this level if a stented aortic prosthesis is already present. When a concomitant aortic valve replacement is planned, it is preferable to place the tricuspid valve sutures before undertaking the aortic valve replacement because tears at this level can be difficult to repair.
PHYSIOPATHOLOGY
Tricuspid valve lesions have traditionally been classified into two groups, organic and functional. These classifications have an important impact on both the type of surgery applied and its long-term results. Organic lesions are those in which the valve apparatus is macroscopically abnormal (See Fig. 79-3B). Functional lesions are those in which regurgitation occurs in the presence of an apparently normal tricuspid valve apparatus (See Fig. 79-3C).
A different classification, proposed by Carpentier,12 distinguishes three main types of disease according to the mobility of the tricuspid valve leaflets: type I, normal leaflet motion with anular dilation; type II, increased leaflet motion due to leaflet prolapse that is secondary to chordal rupture or elongation; and type III, reduced leaflet motion due to leaflet thickening, fused commissures, or leaflet tethering.
Organic Tricuspid Valve Disease
A variety of etiologic factors can induce organic regurgitation, but today, the most frequent cause of organic tricuspid valve disease in urban populations is infective endocarditis (Table 79-1). Tricuspid valve endocarditis used to be relatively rare, with an incidence of only 5% to 10% of patients with infective endocarditis. However, its frequency has dramatically increased with the spread of intravenous drug abuse.13 In this population, the tricuspid valve usually has no preexisting pathologic change. The lesions vary from isolated vegetations to total destruction of the valve, including the anulus. Staphylococcus aureus remains the most common organism found in drug addicts, followed by gram-negative organisms and Candida. Fungal infections are also increasing because of longer periods of invasive monitoring of patients with multiorgan failure in intensive care units.
Table 79–1 Causes of Organic Tricuspid Valve Disease
In the developing world, rheumatic fever is the primary cause of organic valvular heart disease. Typical lesions show varying degrees of leaflet thickening and (most often) commissural fusion. In severe cases, the thickened leaflets become diaphragm-like, with a central circular orifice (see Fig. 79-3B). The subvalvular apparatus is seldom affected, and calcifications are rare. Although tricuspid valve stenosis is the classic lesion, predominant insufficiency is just as common. In a series of 253 patients with rheumatic heart disease who underwent tricuspid valve surgery, we found that organic involvement was present in 45% of the cases and that 45% of them also had anulus dilation.14 In a classic study of 100 postmortem hearts with rheumatic disease, Gross and Friedberg15 found microscopic evidence of inflammation in the anulus of all four valves (in the acute rheumatic attack). Rheumatic tricuspid valve disease is always associated with rheumatic mitral valve or mitral-aortic valve lesions. The incidence of chronic rheumatic tricuspid valve disease associated with rheumatic mitral valve disease varies widely, from 6% in an echocardiographic study16 to 33% in an anatomic series17 and 11% in our series of 1052 patients undergoing rheumatic valvular surgery.14 In a Mayo Clinic surgical pathology study of excised tricuspid valves at the time of valve replacement,18 postinflammatory etiology was responsible for 53% of the 363 valves studied. However, this frequency had diminished from 79% during the period from 1963 to 1967 to 24% during 1983 through 1987, reflecting the reduction in the incidence of rheumatic fever in the United States.
Leaflet tears and total or partial avulsion of a papillary muscle head occur after closed chest trauma. They are occasionally diagnosed at surgery and only classified postoperatively as traumatic by the patient, who recalls an old accident when prompted by the surgeon.19–21
An occasional cause of traumatic tricuspid regurgitation is that induced by the bioptome during a right myocardial biopsy in transplanted patients. Leaflet tears or chordal avulsion results in severe regurgitation that requires urgent surgery.22
Degenerative tricuspid regurgitation associated with mitral valve prolapse is being increasingly observed. This double valve lesion is particularly frequent in Marfan syndrome as a manifestation of a fibrillopathy that also involves the aortic valve and ascending aorta. The reported frequency of tricuspid valve involvement among patients with mitral valve myxomatous disease oscillates between 21% and 52%.7,23,24
Less common causes include organic tricuspid valve lesions secondary to carcinoid syndrome and appetite-suppressant drugs.25 In both cases, the leaflets are encased by a fibrous sheath that reduces their mobility, resulting in stenotic and regurgitant lesions. Other rare causes are listed in Table 79-1.26–28
Functional Tricuspid Regurgitation
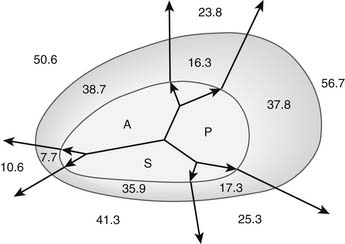
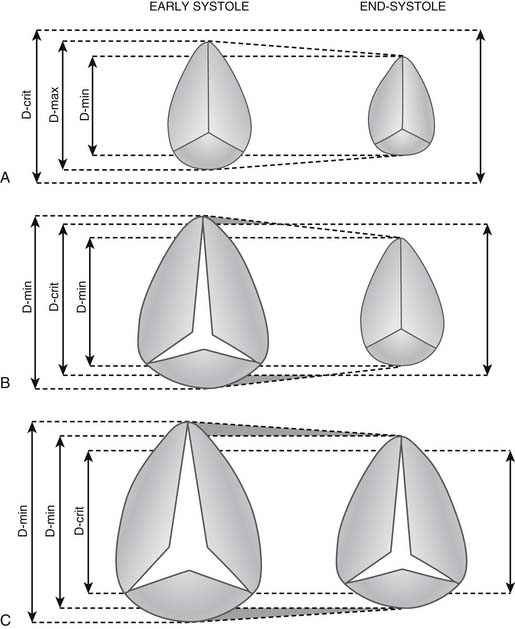
Functional tricuspid insufficiency is understood to be exclusively due to anulus dilation and dysfunction (see Fig. 79-3C). The leaflets, chords, and papillary muscles are otherwise normal. Because of the lack of an anatomic fibrous anulus, the tricuspid valve’s anulus follows the dilation of the right ventricle. The total perimeter of the normal anulus is approximately 100 to 120 mm. In cases of functional tricuspid regurgitation, the circumference of the anulus can reach 150 to 170 mm.29,30 This anulus dilation is nonhomogeneous. In a postmortem study that included normal controls and hearts with rheumatic or myxomatous tricuspid valve disease, Carpentier and colleagues31 showed that the anterior and posterior segments of the anulus dilated far more than the septal portion of the anulus (Fig. 79-5). This report formed the basis for all anuloplasties that selectively reduce the whole anulus except at the level of the septum.
In congestive heart failure, functional tricuspid regurgitation is a predictor of poor survival32 and may be an independent risk factor for the development of cardiac cachexia and protein-losing enteropathy.33 Koelling34 studied a total of 1436 patients with left ventricular systolic dysfunction (ejection fraction <35%). Mitral regurgitation was moderate in 30% and severe in 19%. Moderate tricuspid regurgitation was present in 23% and severe in 12%. Patients with severe mitral regurgitation were more likely also to have tricuspid regurgitation.
Advances in echocardiography have revealed another subvalvular mechanism that applies to both mitral and tricuspid functional regurgitations. Originally observed in functional ischemic mitral regurgitations, it also applies to the ischemic tricuspid valve and most probably to all functional insufficiencies. Right (or left) ventricular remodeling induces a lateral displacement of one or more papillary muscles that apically pulls (through the basal chords) the body of the corresponding leaflet. The leaflet becomes tethered down, losing its coaptation.7,35
Functional tricuspid regurgitation is an expression of right ventricular failure with a generalized distortion of ventricular geometry. Although anuloplasty can reduce or abolish functional tricuspid regurgitation (by reducing the dilated anulus), it does not address the altered subvalvular geometry. Awareness of this important mechanism is giving rise to new and exciting surgical approaches directed toward redressing the geometric distortion.36
DIAGNOSIS
It is imperative to be aware that underestimation and overestimation of the regurgitant jet area are possible because of changes in gain, filter settings, angle, and distance of the transducer. Experience is required to gain a three-dimensional concept of the direction, location, size, and number of regurgitant jets.37 Color Doppler study can even discover minimal regurgitations in 60% to 100% of the normal population.38 These “physiologic” regurgitations are useful to evaluate right ventricular and pulmonary pressures.39 Contrast echocardiography is a reliable method for detecting tricuspid regurgitation40,41 and a patent foramen ovale.
Semiquantitative evaluation of the severity of tricuspid regurgitation is based on the degree of penetration of the regurgitant jet into the right atrium. A jet that penetrates 2 cm into the right atrium indicates mild regurgitation. A jet that penetrates 3 to 5 cm is considered moderate regurgitation; if it is accompanied by systolic flow reversal of hepatic or caval veins, it is considered severe regurgitation. A more quantitative assessment of the degree of tricuspid regurgitation is performed by the orifice flow acceleration proximal isovelocity surface area (PISA) radius method. A radius of 1 to 4 mm indicates mild regurgitation; 5 to 8 mm indicates moderate regurgitation; and greater than 9 mm indicates severe regurgitation.42 These methods are considered essential in the evaluation of mitral regurgitation, particularly in the intraoperative evaluation of residual regurgitations after repair. Unfortunately, they are seldom applied to the tricuspid valve.
Knowledge of the tricuspid valve anulus diameter is important for the surgeon, but its echocardiographic measurement is difficult because the anulus is not circular. Small variations in the orientation of the ultrasound beam can provide very different measurements. Search for a “critical anulus diameter” beyond which the tricuspid valve should not be ignored by the surgeon has been ongoing. Ubago and associates30 suggested 27 mm/m2 as the “critical diameter” above which functional regurgitation always appeared (Fig. 79-6). Using transthoracic echocardiographic examination of 11 consecutive patients with severe clinical tricuspid regurgitation, Come and Riley43 reported a nonindexed mean diastolic anulus diameter of 51 mm in the four-chamber view and 54 mm in the short-axis view. Among 15 controls, the mean anulus diameter was 34 mm in the four-chamber view and 33 mm in the short-axis view. Goldman and colleagues41 suggested 30 mm as the cutoff point between absent or mild regurgitation and moderate to severe regurgitation.
A cutoff point must be found beyond which an anuloplasty will fail. Fukuda and associates,44 in a preoperative and postoperative echocardiographic study of 216 patients with functional tricuspid regurgitation, found that age, tethering height (distance between anulus plane and leaflet coaptation point), and severity of regurgitation were independent parameters predicting residual regurgitation. Preoperative anulus dimension was not associated with outcome of tricuspid valve anuloplasty. In practical terms, it is safe to consider a nonindexed anulus size beyond 40 mm an indication for surgery. When echocardiographic data are not available or are considered unreliable, Dreyfus and coworkers45 suggested using a ruler to directly measure the maximum stretched orifice from the anteroseptal to anteroposterior commissures. Those patients with a dimension greater than or equal to 70 mm (circumference ± 140 mm, diameter ± 44 mm) should undergo anuloplasty.
Preoperative differentiation between organic and functional tricuspid valve disease is also possible. Transvalvular gradients, leaflet irregularities, thickening, and doming are clear indications of organic disease. Transvalvular gradients calculated with continuous wave Doppler echocardiography have been shown to correlate well with cardiac catheterization.46 The normal mean gradient is less than 2 mm Hg, and the end-diastolic gradient is nearly zero. Significant stenosis of the tricuspid valve may be present with a mean gradient of 3 to 5 mm Hg and an end-diastolic gradient of 1 to 3 mm Hg.42 Anulus dilation is most often present in both organic and functional lesions, but it is larger in functional regurgitation.29,47
Recently, three-dimensional echocardiography (although not widely available) is enlarging our knowledge of the geometric changes in the normal and diseased tricuspid valve. It is already providing invaluable information on the anulopapillary complex.7,48