Surgical Treatment of Non-Small-Cell Lung Cancer
Joseph LoCicero III
Using statistics from the National Cancer Institute’s (NCI’s) Surveillance Epidemiology and End Results (SEER) data, Ries et al.231 reported that 215,020 individuals (114,690 men and 100,330 women) will be diagnosed with cancer of the lung/bronchus and 161,840 will die of it in 2008 (Table 110-1). This incidence has risen markedly since 2003, when only 170,000 people were diagnosed with lung cancer. From 2001 to 2005, the median age at diagnosis for cancer of the lung and bronchus was 71 years. None were diagnosed under age 20; approximately 0.2% between 20 and 34; 1.9% between 35 and 44; 8.8% between 45 and 54; 21.0% between 55 and 64; 31.9% between 65 and 74; 28.9% between 75 and 84; and 7.3% at >85 years of age.
The prevalence of non-small-cell lung cancer in the United States as of January 1, 2005, was chronicled in the same communication. There were approximately 360,081 individuals alive who had a history of cancer of the lung and bronchus: 172,426 men and 187,655 women. The probability of an American developing lung cancer increases with age: 0.020% at age 40, 0.185% at age 50, 0.487% at age 60, 1.304% at age 70, and nearly 2% at age 80.
Five-year survival, while still dismal for all patients with lung cancer, has risen steadily since the NCI began keeping statistics. For patients diagnosed in 1976, the 5-year survival was 11.9%. For those patients diagnosed in 2000, the survival rate is 16.2%. The main reason for such poor survival is that, as Kobrinsky and colleagues135 point out, over 40% of patients regardless of smoking history present with metastatic disease at the time of diagnosis. For comparison, breast, prostate, and colon cancers—which have prevalence rates similar to those of lung cancer—present at a much earlier stage (Table 110-2). Around 5% of patients with breast cancer, 17% of patients with prostate cancer, and 21% of patients with colorectal cancer present with metastasis.
For the minority of patients (30%) with non-small-cell lung cancer (NSCLC) limited to the lung, pulmonary resection as the primary or the sole treatment remains the most effective therapy. For the 28% of patients with more advanced locoregional involvement, researchers continue to juggle a multimodality approach that may or may not include resection. The growing experience with combining operation with other treatment (adjuvant and induction therapy) is discussed in Chapter 117.
Historical Aspects
Surgical resection for lung cancer began with the first successful pneumonectomy, reported by Graham and Singer92 in 1933. Subsequent advances have led to smaller resections and improved operative mortality rates. Bronchoplastic procedures were developed during the late 1940s, culminating in Allison’s successful sleeve lobectomy for a bronchial carcinoma in 1952, as reported by Price-Thomas.218 Lesser resections, such as lobectomy and segmentectomy, were pioneered in the 1940s. Churchill and Belsey38 in 1939 demonstrated the feasibility of segmentectomy in a patient with bronchiectasis. These techniques were developed and popularized by Overholt and associates198 in 1946; they were reintroduced by Jensik and colleagues123 in 1973 and by Shields and Higgins.260 The introduction and refinement of surgical staplers have made lung resection safer, faster, and less traumatic while maintaining surgical oncologic principles. Thoracoscopic approaches, in conjunction with staplers, have spawned hybrid techniques such as the extended segmentectomy reported by Atkins and colleagues15 in 2007, and extended wedge resections, as reviewed by Raz and associates.223
Overview of Resection for Non-Small-Cell Lung Cancer
Every patient with locoregional NSCLC should be approached as a potential candidate for resection. At present, most patients with clinical stage I and II NSCLC undergo resection as the definitive primary therapy. However, most patients with stage II and a subset of patients with stage Ib are offered adjuvant chemotherapy. Most patients with clinical stage IIIA or IIIB should not be resected primarily but rather considered for multimodality therapy, ideally as part of a clinical trial. Only under exceptional circumstances should patients with stage IV NSCLC be considered for resection. Certain anatomic or physiologic
considerations may make an individual patient a poor candidate for resection.
considerations may make an individual patient a poor candidate for resection.
Table 110-1 ESTIMATED NEW CANCER CASES AND DEATHS FOR 2008 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 110-2 Cancer Stage at Diagnosis and Smoking History | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Anatomic Considerations
Complete resection is the goal of all operations for lung cancer. There should be a logical progression of staging from chest radiography to computed tomography (CT) of the chest and abdomen and other imaging to invasive assessment, most commonly surgical mediastinal evaluation (Chapter 18) and occasionally biopsy of extrathoracic sites, and, if indicated, to thoracic exploration. A detailed discussion of radiologic evaluation appears in Chapters 9, 10, 11, 12 and 13. In patients in whom the tumor is deemed resectable, the appropriate procedure is carried out. If a tissue diagnosis is not made preoperatively, an operative biopsy is mandatory, particularly if more than a lobectomy is required.
When the clinical and imaging data indicate that a complete resection cannot be achieved or that the clinical tumor stage is associated with a poor long-term outcome even with complete resection, operation is not indicated as initial therapy. Induction therapy before an attempted resection should be considered, or resection should be abandoned as an option. Tumors that present as T4 lesions due to involvement of vital structures are mostly unresectable. However, small numbers of carefully selected patients with involvement of the superior vena cava, aorta, and left atrial–pulmonary vein confluence appear to benefit from operation, usually as part of a combined approach. Patients with phrenic or recurrent laryngeal nerve invasion, once considered an absolute contraindication to resection, are being included in multimodality protocols leading to operation. Patients with T3N0 (stage IIB) NSCLC due to chest wall invasion or carinal proximity are candidates for initial complete resection. In addition, a small number of centers have accumulated substantial experience with operation for selected cases of “unresectable” T4 lung cancer, such as those with tracheal involvement.
At the extremes are T4 tumors due to additional tumors or satellite nodules in the same lobe or malignant pleural effusion. Resection of tumors with satellite lesions in the same lobe is acceptable. Operation in cases of T4 cancer due to malignant pleural effusion, in contrast, offers no survival advantage over less invasive therapies. It is imperative to note that reports of successful surgery in locally advanced cancers constitute a truly minuscule fraction of lung cancers, are generally performed in high-volume centers, and cannot be routinely extrapolated to the overall decision pathway for NSCLC.
The optimal initial management of NSCLC with clinically proven lymph node dissemination is also nonsurgical. N3 disease remains out of bounds for resection. This was pointed out by Watanabe and colleagues,301,302 who support the position that even the anterior mediastinal nodes (station 3) have a dismal prognosis. In addition, little disagreement remains for patients with N2 disease documented before thoracotomy (clinical N2). Such patients are candidates for multimodality protocols such as the SWOG 8805 trial reported by Albain and associates4 in 1995. This topic is covered in detail in Chapter 117.
In contrast, patients discovered to have N2 disease, particularly single-station N2 disease, at thoracotomy following an appropriate negative invasive evaluation (clinical N0–1, pathologic N2), should undergo resection at that time. Careful consideration, however, should be given before performing a pneumonectomy in the setting of N2 disease with obvious extracapsular involvement. Resection as primary treatment in the patients without bulky lymphadenopathy found at operation carries a better prognosis than for patients with prethoracotomy N2 status. Long-term survival rates are between 17% and 28%, as first reported by Pearson207 and confirmed by Daly42 and D. L. Miller177 and their colleagues, among many others. The experience with postoperative adjuvant therapy in these patients is detailed in Chapter 116.
Metastatic Disease
The presence of multiple extrathoracic metastases is almost always an absolute contraindication to pulmonary resection. When a solitary metastasis is thought to be present, resection of the primary lung cancer may be considered only in occasional cases of cerebral metastasis and only after thorough imaging and invasive assessment have confirmed the absence of other sites of disease. Multiple series have shown compelling evidence of the efficacy of complete resection, when possible, of both the cranial and intrathoracic sites. In contrast, median survival with no treatment, steroids only, or cranial radiation is about 1 month, 2 months, and 6 to 9 months, respectively. Although a handful of synchronous solitary adrenal metastases have been treated by combined resections, experience with this approach is limited, and an initial nonsurgical plan is preferable. Except in unusual circumstances, primary lung resection is not indicated in the presence of other sites of dissemination, even if clinically thought to be isolated.
In all cases of suspected single metastasis associated with limited intrathoracic disease, one must ensure that a distant lesion is solitary and that it is malignant before deciding against primary pulmonary resection. Brain metastases are usually diagnosed reliably by imaging and rarely require invasive confirmation. If an apparently solitary cerebral metastasis is identified by CT scan, however, it is often prudent to obtain a magnetic resonance imaging (MRI) scan of the brain before resection because of the superior sensitivity of the latter technique. Additional imaging is also often required after abnormalities are detected on bone scan or after an adrenal, renal, hepatic, or other mass is identified by CT. In most instances, a synthesis of the data provided by an appropriate combination of plain radiographs, CT, MRI, ultrasound, and positron emission tomography (PET) will reliably confirm or rule out distant metastasis. In the few patients with solitary lesions that remain equivocal, percutaneous or open biopsy may be indicated before pulmonary resection is denied. The importance of securing a reliable radiographic or tissue diagnosis in this setting is exemplified by the findings of Porte and associates,216 who reported that nearly half of all adrenal masses detected during the evaluation of NSCLC patients were benign. In general and if reasonable, the metastasis is resected first, followed as soon as feasible by the lung resection.
Physiologic Considerations
Traditionally established physiologic barriers to surgical resection have been falling steadily over recent years. Centers with a strong commitment to aggressive preoperative and postoperative care are reporting mortality and morbidity rates equal to those of series with limited patient entry.
Age
Older age per se is not a contraindication to resection. Ng and colleagues192 in 2005 reported that advanced age as a contraindication to surgery no longer exists in major medical centers. Naunheim and associates191 reported good survival among octogenarians having a pneumonectomy. Pagni and colleagues200 performed 24 pneumonectomies in patients >70 years of age with a 12.5% operative mortality rate, compared with a 4.3% mortality rate for pneumonectomies in younger individuals.
Of more concern is the denial of surgical intervention to seniors with disabilities, as pointed out by Iezzoni et al.112 in 2008. They found that Medicare beneficiaries with disabilities were much more likely to be male, non-Hispanic black, and not currently married. And, although 82.2% of nondisabled persons had surgery, only 68.5% of disabled persons did so. But statistically significant cancer-specific mortality differences disappeared after accounting for these treatment differences. Iezzoni et al.112 therefore urged careful evaluation of all seniors in terms of a surgical option.
Long-term survival following resection is not different for elderly patients, as pointed out by Harvey101 and Ishida118 and their colleagues. However, there tends to be a decreased survival in patients <40 years of age, as noted by Pemberton and coworkers.208 Jubelirer and Wilson125 attribute this difference to more advanced disease at the time of diagnosis rather than to a differential response to treatment.
Ageism does continue to exist in the oncologic community. The issues were clearly enumerated by Bouchardy and associates,23 who, in 2007, discussed the underrepresentation and undertreatment with chemotherapeutic agents of elderly women with breast cancer. They described both objective and subjective reasons. Objective reasons at the origin of undertreatment were, notably, a higher prevalence of comorbidity, lowered life expectancy, absence of data on treatment efficacy in clinical trials, and increased adverse effects of treatment. More subjective reasons were putative lowered benefits of treatment, potentially less aggressive cancers, social marginalization, and physician’s beliefs. As bronchioloalveolar cell lung cancer becomes more prevalent, similar concerns may surface among oncologists treating lung cancer.
Cardiac Disease
Despite the fact that most patients with lung cancer are older and have a significant smoking history and other risk factors that lead to coronary artery disease, significant cardiac morbidity is rare in patients undergoing a thoracotomy for carcinoma. A myocardial infarction within 3 months before surgery carries some risk for reinfarction. However, Rao and associates220 found this rate to be only 5.7%. A full discussion of the appropriate cardiac evaluation for thoracotomy is covered in Chapter 21. If significant disease is identified, many patients can still undergo pulmonary resection, as reported by Piehler and associates.207 Successful resections have been performed after angioplasty, concomitantly with coronary artery bypass surgery or sequentially within 2 weeks after bypass.
Some patients with active coronary ischemia will have lung cancer and require resection. Current controversies over coronary artery stenting with a bare metal stent or a drug eluting stent require careful coordination between the team treating the lung cancer and the cardiologists. The latest recommendations suggest that, if the patient requires a stent in preparation for a lung resection, a bare metal stent be placed. The patient is at significant risk for the first 3 to 6 weeks after placement and should remain on aspirin and clopidogrel. At that point, clopidogrel may be stopped 5 days before the planned procedure. However, at day 3, the practice of substituting a short-acting antiplatelet agent such as a small-molecule 2b3a inhibitor for clopidogrel should protect the coronary artery lesion and prevent excessive bleeding at the time of surgery. Dual antiplatelet therapy should
begin on postoperative day 1 once the patient’s bleeding is considered under control. For more details, see Chapter 21.
begin on postoperative day 1 once the patient’s bleeding is considered under control. For more details, see Chapter 21.
Pulmonary Function
Poor measured pulmonary function has traditionally been considered a formidable barrier to resection. Based on old unconfirmed data, elaborate schemas were established to filter only the best candidates for resection. We have learned from the experience with surgery to reduce lung volume that nearly all patients will tolerate some type of pulmonary resection. In general, individuals who function daily at a normal activity level, regardless of their measured parameters, will do well. A report by Korst and coworkers140 showed that after 6 months, patients who underwent a lobectomy had measured pulmonary function that was not statistically different from their preoperative values. In fact, some patients who had upper lobectomies actually had better postoperative function. Nonetheless, pulmonary function testing (PFT) remains an important objective evaluation of a patient’s ability to tolerate resection. However, no single parameter has proved to be reliably prognostic, and strict interpretation could deny resection to many physiologically eligible patients. Of most importance is that the pronouncement that “patients require a postoperative residual FEV1 of 800 mL,” made by Gaensler and associates80 in 1955 and heavily quoted to this day, should be expunged from the literature of the third millennium.
Algorithms for preoperative pulmonary evaluation are presented in Chapter 20. Briefly, assessment begins with the patient’s history, including the patient’s physical activity. Specifically, examples of cardiopulmonary fitness include the ability to climb one or more flights of stairs, the ability to walk more than a block without stopping, and the ability to perform house or yard work without difficulty. Office assessment of exercise capacity may include a modified 6-minute walk test.
Standard PFTs should be performed and include the following: spirometry, including forced vital capacity (FVC), forced expiratory volume in one second (FEV1), and forced expiratory flow rate (FEF); and lung volumes, including total lung capacity (TLC), residual volume (RV), functional residual capacity (FRC), and diffusing capacity of the lung for carbon monoxide (DLCO). The prime values are the FVC, which is a surrogate for restrictive disease; the FEV1/FVC ratio, which is a dimensionless variable denoting the patient-specific obstructive disease; and the DLCO, which is a surrogate for oxygen diffusion defects.
The predicted postoperative parameters (e.g., PPFEV1) can be estimated by multiplying the measured FEV1 by the expected number of segments remaining after resection, each of which is assigned a contribution to overall pulmonary function of 5%. A PPFEV1 of less than 30% of the patient’s expected value may be cause for concern. Additional testing that may be helpful in equivocal situations includes quantitative ventilation/perfusion scanning and cardiopulmonary exercise testing.
Principles of Surgery for Non-Small-Cell Lung Cancer
The goal of surgical treatment of NSCLC is complete resection. Incomplete resection leaving visible tumor behind (R2) impairs the patient’s quality of life. Incomplete resection confers no therapeutic advantage. Moreover, R2 resections cause unnecessary pain and suffering and temporally postpone any potential benefit of subsequent radiation therapy and/or chemotherapy. With currently available staging modalities, the incidence of aborted exploratory thoracotomy or grossly incomplete resections should be minuscule.
In contrast, despite appropriate preoperative assessment, an R1 resection leaving a microscopically positive resection margin (usually involving the bronchial margin), a finding of subclinical parenchymal sites of cancer, or, most commonly, the pathologic documentation of unsuspected malignant lymphadenopathy, is often unavoidable. In addition to the absence of gross residual tumor and microscopically negative surgical margins, some believe the definition of a complete resection should include negativity of the highest or most distant resected lymph node. However, this has never been proven.
Every operation for lung cancer has three essential parts: establishment or confirmation of the diagnosis and the intrathoracic stage, complete resection of the tumor, and the systematic sampling or complete dissection of all ipsilateral lymph node stations potentially draining the primary tumor.
Intraoperative Diagnosis and Surgical Staging
Depending on the specifics of each case and the surgeon’s preferred approach, a diagnosis of NSCLC will have been made preoperatively in many patients by bronchoscopy or transthoracic needle biopsy, less commonly by thoracoscopy, and rarely by mediastinoscopy in cases of primary resection. When a diagnosis of cancer has not been secured before thoracotomy, either intentionally or because of inconclusive results, it is advisable to establish a diagnosis intraoperatively before proceeding to anatomic resection. Stapled wedge resection and frozen section will most often accomplish this goal. Frozen section assessment for NSCLC is generally straightforward and accurate. For lesions that are central or otherwise not amenable to a parenchyma warping wedge resection, sampling can be carried out by fine-needle aspiration (FNA) cytology or core needle biopsy. Incisional biopsy may occasionally be needed but is less desirable because it requires macroscopic violation of a potential neoplasm. For the same reason, stapling across abnormal tissue for diagnostic assessment is discouraged. In the rare instance of a tumor of uncertain behavior not amenable to wedge resection and in which aspiration or core biopsy is nondiagnostic, a lobectomy may be required. Pneumonectomy and extended lobar resections, however, should not be performed in the absence of a firm confirmation of malignancy.
In addition to confirming the diagnosis of cancer when necessary, exploration of the chest for NSCLC includes an assessment of the hemithorax for other sites of disease, whether suggested by preoperative imaging or not. The entire lung is palpated and inspected for other masses. Sequentially, fixation of the primary tumor to adjacent structures is assessed, followed by the pleura for metastasis and the hilar and mediastinal nodes. Although it is rare to abandon a planned resection based on unexpected intraoperative findings, an occasional seemingly straightforward case may become unresectable.
In patients with unexpected, small malignant pleural effusions found at thoracotomy, Sawabata and associates253 reported very poor survival, with a median survival time of 13 months and
5-year survival rate of 9%, even when the resection was complete. There is no current consensus, however, regarding how to proceed in this setting. Ideally, rapid cytology is performed on the fluid. If positive, a decision regarding resection is made based on the total clinical situation. Extensive parenchymal resections should not be done if the fluid is known to be malignant.
5-year survival rate of 9%, even when the resection was complete. There is no current consensus, however, regarding how to proceed in this setting. Ideally, rapid cytology is performed on the fluid. If positive, a decision regarding resection is made based on the total clinical situation. Extensive parenchymal resections should not be done if the fluid is known to be malignant.
Resection generally should proceed when unsuspected lymphadenopathy is found at operation after appropriate invasive and noninvasive staging evaluation. Exceptions include the unusual finding of unexpected extensive, fixed, or “bulky” adenopathy. Also, in a physiologically marginal patient, pneumonectomy should not be done in the presence of positive interlobar nodes or direct tumor extension across major fissures. In any case, there should be no hesitation to sample and assess by frozen section or cytologic analysis any nodal, pleural, or parenchymal tissue or pleural fluid that, if positive, would render resection inappropriate.
The potential for incomplete resection is generally known preoperatively and should be discussed clearly with the patient. Although there is no specific modern benchmark, the incidence of aborted thoracic exploration in older series was as high as 20%; currently, it should be 1% or less. Despite appropriate clinical staging, a small number of patients with locally advanced lesions still require exploratory operation to ascertain resectability with certainty.
Completion of lymph node staging should be part of a planned pulmonary resection. Data from the American College of Surgeons Oncology Group, reported by Allen and colleagues9 in 2006, demonstrate that a complete lymphadenectomy does not add a significant amount of operative time and does not add to the overall morbidity of the procedure. Such an approach will yield the most accurate staging of a patient’s lung malignancy.
In addition to standard surgical staging by gross inspection and frozen section, a number of authors, including Kondo et al.139 in 1993 and Nakagawa and colleagues185 in 2007, have suggested that intraoperative pleural lavage cytology be performed in all cases without obvious pleural effusion, before resection or prior to chest closure, or both. The rationale for this procedure is to detect the presence of malignant cells in the pleural cavity and thereby identify patients at higher risk for recurrence. As noted by Kotoulas and coworkers,141 positive cytology tends to be associated with a more advanced primary T factor. Although the volume of lavage fluid employed varies, all protocols require that the preresection sample be obtained immediately upon entry into the chest, before any manipulation of the tumor. When obtained, the postresection sample is taken just before closure of the thoracotomy, with some protocols requiring prior copious pleural irrigation. Higashiyama and associates103 found pleural recurrence in 26% of patients when both the initial and preclosure samples were positive.
Rami-Porta and colleagues,219 reporting for the International Association for the Study of Lung Cancer lung cancer staging project (seventh edition), proposed that positive pleural fluid along with carcinoma in situ at the bronchial margin be classified as “uncertain resection.”
Resection
After a diagnosis of cancer has been made and resectability established, the appropriate pulmonary or extended resection, along with systematic lymph node sampling or lymphadenectomy, is carried out. For patients with adequate lung function, the current standard cancer resections include lobectomy, bronchoplastic lobectomy, bilobectomy, and pneumonectomy, based on the extent of disease. In some cases, an anatomic segmentectomy may be appropriate. At present, nonanatomic or “wedge” resection should be considered as definitive therapy only in the minority of patients whose cardiac or pulmonary status mandates conservation of pulmonary parenchyma or in certain cases of synchronous or metachronous multiple tumors. There is increasing interest is assessing limited operations for NSCLC that is considered low-grade by preoperative imaging such as “ground glass” opacity on CT scan (see Chapter 104). Although summarized subsequently, the morbidity and mortality of the various resections are discussed in detail in Chapters 28, 29, 30, 31, 32, 33, 34, 35 and 36.
Lobectomy
Lobectomy is the ideal operation for resection of a lung cancer confined to the parenchyma of a single lobe. It permits removal of the tumor along with the associated peripheral (pleural) and central lymphatic drainage pathways. Lobectomy is generally well tolerated, usually leaves sufficient lung volume to fill the pleural void left by resection, and avoids some of the short-term and late complications of pneumonectomy. Lobectomy is associated with about half the operative mortality of pneumonectomy (about 2% versus 4%), as reported by Ginsberg85 and Wada295 and their associates. Pagni and associates200 reported operative mortality rates for lobectomy: 2% in 293 patients >70 years of age and 4% in 45 octogenarians. Schneider and colleagues256 in 2008 demonstrated a mortality rate of 1.9% in patients >75 years of age and a long-term survival no different from that in patients between 65 and 75 years of age.
Bilobectomy.
A bilobectomy involves resection of the right upper and middle lobes or of the right lower and middle lobes. The former operation is indicated when a tumor located in the anterior segment of the right upper lobe or in the right middle lobe has spread across the minor fissure or approximates an incomplete fissure. Failure to perform a bilobectomy in this setting may result in a positive or unacceptably close parenchymal resection margin, or failing to resect the drainage system of the invaded segment. When a tumor in the right lower lobe is central, a bilobectomy may be required because of the proximity of the origins of the superior segmental and middle lobe bronchi. Other indications may include certain cases of interlobar vascular or nodal involvement, but a pneumonectomy should be considered in such instances. In a series of 166 bilobectomies reported by Keller and colleagues,131 the indications for this procedure were tumor extending across a fissure in 45%, absent fissure in 21%, endobronchial tumor in 14%, external or nodal invasion of the bronchus intermedius in 10%, vascular invasion in 5%, and miscellaneous reasons in 5%. The operative mortality for bilobectomy is generally reported as higher than for lobectomy but lower than the risk associated with pneumonectomy.
Sleeve Lobectomy
A sleeve lobectomy consists of the resection of a lobe along with a circumferential segment of the adjacent mainstem bronchus; it is generally an alternative to pneumonectomy. Bronchial
continuity is restored and lung parenchyma preserved by anastomosis of the proximal and distal bronchial resection edges. This operation is most often indicated for endobronchial tumors at the origins of the right- or left-upper-lobe bronchi.
continuity is restored and lung parenchyma preserved by anastomosis of the proximal and distal bronchial resection edges. This operation is most often indicated for endobronchial tumors at the origins of the right- or left-upper-lobe bronchi.
Occasionally, sleeve lobectomy is suitable for patients with limited nodal disease affixed to the bronchial wall at the orifices of these lobes. Nodal disease of this type was the indication in 21% of the cases reported by Deslauriers and colleagues.57 Overall, these authors achieved a complete resection in 87% of 142 sleeve lobectomies, with an operative mortality rate of only 2.5%. Their 5- and 10-year survival rates were 63% and 52%, respectively, for stage I tumors. Local recurrence was ultimately seen in 23% overall and in 17% of completely resected cases.
In 2007, Yildizeli of Dartevelle’s group316 reported on 218 patients. They were able to perform a complete resection in 95.9% of the series. Their operative mortality and the morbidity rates were 4.1% and 22.9%, respectively. Multivariate analysis showed that risk factors for mortality and morbidity were compromised patients (p = 0.001), current smokers (p = 0.01), right-sided resections (p = 0.003), bilobectomy (p = 0.03), squamous cell carcinoma (p = 0.03), and presence of N1 or N2 disease (p = 0.01). Their overall 5-year and 10-year survival rates were 53% and 28.6%, respectively. After complete resection, recurrence was local in 10 patients, mediastinal in 20, and distant in 25. By multivariate analysis, two factors significantly and independently influenced survival: nodal status (N0–N1 versus N2; p = 0.01) and the stage of the lung cancer (stage I–II versus III, p = 0.02).
Inferred from these series are the following criteria for optimum results of sleeve lobectomy. Patients should have adequate pulmonary function. The tumor should be limited to the lung so that a complete resection may be accomplished. Patients with negative mediastinal nodes have the best survival. Patients with marginal pulmonary function, tumors invading structures outside of the lung, or positive nodes in the mediastinum technically may be resectable with a sleeve lobectomy but have higher complication rates and shorter survivals than those without these findings.
Sleeve resection of the pulmonary artery can be accomplished with or without a bronchial sleeve resection, but most cases with this degree of local invasion are inoperable or are treated by pneumonectomy. Ma and colleagues154 reported in 2007 on a meta-analysis of studies of pulmonary artery resection. Twelve studies met the defined criteria, including a total of 2,984 subjects having a sleeve resection, but only five studies including pulmonary artery resection. For pulmonary artery resection with sleeve lobectomy, the weighted mean operative mortality was 3.3%, and the complication rate 32.4%. The estimated combined hazard ratio for overall survival in 10 studies was 0.70 (95% CI: 0.62–0.79) in favor of sleeve lobectomy. The median overall survival was 26 months for pneumonectomy, 60 months for the sleeve lobectomy alone, and 30 months for pulmonary artery resection and sleeve lobectomy.
Bronchoplastic Procedures.
It is very hard to evaluate the use of bronchoplasty alone as an adjunct to complete resection, since any bronchus closed with sutures instead of a stapler may be called technically a bronchoplasty. It permits the resection of a little extra bronchus without having to resort to a sleeve lobectomy. A bronchoplastic resection is less often appropriate when bronchi other than those of the upper lobes are involved by NSCLC, but is occasionally undertaken in oncologically favorable situations or as an alternative to pneumonectomy in patients with limited lung function. The success of bronchoplastic resection in properly selected patients is also in the series reported by Watanabe and colleagues,300 with late survival of 79% in stage I, 55% in stage II, 30% in stage III, and 45% overall.
Although concern has been raised that the local recurrence rate is higher following bronchoplastic lobectomy than after pneumonectomy, reports from centers with significant experience with this approach—including those of Vogt-Moykopf,294 Tedder,280 and Deslauriers57 and their associates—show an acceptable operative mortality, a high rate of complete resection, and a late survival that is generally comparable, stage-for-stage, with that of other types of complete resection in NSCLC.
Pneumonectomy
A pneumonectomy is required when a lobectomy or one of its modifications is not sufficient to remove all locoregional disease. It must be kept in mind that a pneumonectomy is a radical procedure that can result in the loss of more than 50% of a patient’s lung function and pulmonary vascular bed. The indications are central tumors that involve the main bronchus, large parenchymal cancers that violate the fissures or invade the interlobar vessels, or hilar lymph node involvement. Pneumonectomy in the latter situation should be reserved for cases in which higher stations are benign and a complete resection is possible. The operative mortality for pneumonectomy is about twice that of lobectomy. Wada and associates295 noted a rate of 3% among 590 patients undergoing resection for lung cancer in Japan in 1994. Right pneumonectomy carries a higher risk than left pneumonectomy. An increasing number of patients with N2 disease or central, locally invasive cancers are now being treated by induction therapy. Because of the extent of their disease, a high percentage require pneumonectomy (23% to as high as 53%). Despite the frequent technical difficulty posed by postinduction peribronchial and perivascular fibrosis, operative mortality in this group can be as low as 5%, but ranges up to 15%, as reported from several multicenter trials by Strauss269 and Weiden305 and their associates. Albain and associates6 in 2005 presented the data from the intergroup trial 0139, which randomized 429 patients with T1-3, N2, M0 NSCLC to chemoradiation alone or followed by surgery. They reported a mortality rate of 26% for pneumonectomy, almost all on the right side. But subsequently, in 2006, Daly and colleagues44 showed equal or better mortality for right pneumonectomy. They reported on 30 patients with locally advanced non-small-cell lung cancer who underwent pneumonectomy after 5,940 cGy of radiation and two cycles of etoposide and cisplatin. To minimize post- pneumonectomy pulmonary edema, patients were treated with a protocol that included fluid restriction and 48 hours of mechanical ventilation. Morbidity, mortality, and survival were examined. Of the 30 patients, 18 had right and 12 left pneumonectomies. Death occurred in four patients (13.3%) but in only one (5.6%) after right pneumonectomy.
Extended Pneumonectomy.
There are three types of extended pneumonectomy. The most commonly employed variation is an intrapericardial pneumonectomy, necessitated by encroachment of a central tumor at or near the entry of the
pulmonary vessels (most often the artery central to its branches) into the pericardium. Division within the pericardium may provide both a greater margin of resection and a longer segment for safe transection of the vessel. Although this approach may be associated with a higher incidence of postoperative arrhythmias, the operative risk is not higher than for standard pneumonectomy.
pulmonary vessels (most often the artery central to its branches) into the pericardium. Division within the pericardium may provide both a greater margin of resection and a longer segment for safe transection of the vessel. Although this approach may be associated with a higher incidence of postoperative arrhythmias, the operative risk is not higher than for standard pneumonectomy.
Another modification is a supra-aortic pneumonectomy and involves transecting the left mainstem bronchus more proximal than in a standard left pneumonectomy closer to the trachea above and medial to the aortic arch. This approach is needed occasionally for tumors originating high in the bronchus.
The third variation is the carinal or sleeve pneumonectomy, consisting of resection of the lower trachea, the carina, and a main bronchus and its associated lung (usually the right) with a tracheobronchial anastomosis of the remaining lung. This procedure is indicated for central lesions approximating or involving the carina that appear totally resectable by this approach. Although some earlier series reported operative mortality in as many as one-fourth to one-third of cases, reports by Dartevelle and associates51 and by Mitchell and colleagues179 have achieved mortality rates of 7% and 15%, respectively. The latter authors noted a decrease from 20% to 10% in operative mortality rates between the first and second halves of their series. Porhanov and colleagues215 reported an operative mortality rate of 16% among 231 carinal resections. Some cases in these series were pure carinal resections or lobar and carinal resections. The risk of the less often performed left carinal pneumonectomy is higher than that of the right lung.
Segmentectomy
It is generally agreed that a segmentectomy is an acceptable operation for NSCLC when a patient has limited pulmonary reserve and a small peripheral tumor confined to an anatomic segment. Whether segmental or wedge resection constitute adequate treatment for small peripheral cancers in general or for cancers in which the preoperative radiographic features suggest a low-grade tumor remains under investigation. Although any segment can be removed by anatomic dissection, resections of the upper lobe segments or the superior segments of the lower lobes are performed most commonly. Lingulectomy, although encompassing two segments, is also a form of segmentectomy and is often feasible for peripheral NSCLC.
Jensik and colleagues123 reported the first large series of segmental resection for lung cancer. Among 123 patients, 5-year and 10-year survival rates were 56% and 27%, respectively. In an instructive analysis, Kodama and colleagues136 compared three groups of patients with T1N0 NSCLC: (a) 46 patients undergoing segmentectomy as an intentional procedure, (b) 17 patients in whom segmental resection was viewed as a compromise because of limited lung function, and (c) 77 patients treated by lobectomy and lymph node dissection. There was no significant difference in late survival between the lobectomy group (88%) and the intentional segmentectomy patients (93%). However, the difference in survival between these two groups and patients undergoing segmentectomy as a compromise procedure (48%) was significant. In a report by Warren and Faber297 comparing 68 patients with T1–2N0 tumors treated by segmental resection with 105 similar patients undergoing lobectomy, there was also an overall survival difference favoring the lobectomy group, but the differential was not significant for tumors ≤3 cm in size. However, for the total series, the rate of locoregional recurrence was 23% following segmentectomy, as contrasted with 5% after lobectomy. The only prospective experience, collected by the LCSG and reported by Ginsberg and Rubinstein,87 indicates that local recurrence following limited resection for T1N0 NSCLC (including both segmentectomy and wedge excision) is threefold higher than for lobectomy, although ultimate survival was not significantly different. Despite an increased risk for local recurrence, anatomic segmental resection remains an appropriate option in patients with limited lung function and also in those with small peripheral tumors.
Wedge Resection
In contrast to segmentectomy, wedge resection is a nonanatomic operation that should be considered as definitive therapy only in poor-risk patients. Despite a higher risk of local recurrence when compared with anatomic resection, wedge excision may still be preferable to alternative treatments. In an early nonrandomized series reported by Errett and associates,69 wedge resection was performed as a compromise operation in 97 patients with pulmonary impairment and compared with the outcomes in 100 patients treated by lobectomy. Despite higher predicted risk, the wedge resection group incurred only a 3% operative mortality rate, as compared with 2% in the lobectomy group; late survival was not statistically different. In patients with T1N0 NSCLC, Landreneau and colleagues147 retrospectively compared 42 cases treated by open wedge resection, 60 by video-assisted wedge resection and 117 by standard lobectomy. Despite reduced pulmonary function and older age, there was no mortality in the combined wedge groups, as compared with a 3% mortality rate in the lobectomy group. However, as in the LCSG experience, local recurrence rates were higher in the open and VATS wedge patients (24% and 16%, respectively) than in the cases treated by lobectomy (9%). Although the 5-year survival rate was significantly lower in the open wedge cohort than in the lobectomy patients (58% versus 70%), the 5-year survival rate, at 65%, in the VATS patients was similar to that in the lobectomy group. The authors point out that the minimum requirements for an appropriate wedge resection for NSCLC include the following: a tumor <3 cm in diameter; a location in the outer third of the lung and technically amenable to adequate local excision, absence of endobronchial extension, clear margins by frozen section; and mediastinal and hilar lymph node sampling. When these criteria are met, wedge resection is an acceptable option in the few patients unable to tolerate an anatomic operation. The Cancer and Leukemia Group B (CALGB) has opened a trial of lobectomy versus sublobar resection (segmentectomy or wedge resection) for cancers <2 cm. This trial was approved for accrual in 2007.
With the use of radiation adjuncts, recent trials suggest that long-term results of wedge resection may be enhanced by applying local implanted radiation seeds at the site of resection. The American College of Surgeons Oncology Group (ACOSOG) has established a randomized trial comparing sublobar resection (wedge resection) to sublobar resection plus implanted radiation seeds. This trial opened in 2006 and is expected to close in 2009.
Local excision by cautery or laser has been performed, but the late benefit is unknown, and this approach cannot be recommended as standard.
Minimally Invasive Resection
Thoracoscopic techniques have been successfully employed for lobectomy, pneumonectomy, and local resection of NSCLC. For surgeons with experience and skill with this methodology, thoracoscopic approach is an acceptable alternative to open operation. It is essential that the same principles of lung cancer surgery that guide standard resection be maintained when minimally invasive techniques are applied. This topic is addressed in detail in Chapters 33, 34, and 35.
Alternative Procedures
As an alternative to resection, radiofrequency ablation has been proffered as a reasonable alternative for the marginal patient. Dupuy and associates63 introduced the procedure in 2000 as a natural progression of the technology, as it has been applied to the liver. Sporadic reports since then have shown that the technique is feasible and may be done with minimal morbidity, even in marginal patients. ACOSOG initiated a single armed trial in 2006 to study this modality. The major endpoint is 2-year survival rate.
Evaluation of the Lymph Nodes
The single most important prognostic surgical factor in NSCLC is the status of the mediastinal lymph nodes. Evaluation begins preoperatively with a CT scan of the chest. Although there is controversy regarding the need for routine mediastinal evaluation, all accessible nodes that are enlarged should undergo biopsy before thoracotomy. Nodes that are positive on PET scan, whether or not enlarged on CT, must undergo biopsy to rule out both metastasis and false-positive uptake. In addition, mediastinal node assessment should be considered in all patients at high risk for metastasis, such as those with central tumors. In addition to mediastinoscopy, extended mediastinoscopy, and anterior mediastinotomy (see Chapter 18), endobronchial ultrasound and esophageal ultrasound are often useful (see Chapter 14). Thoracoscopy is no longer indicated as a method of primary mediastinal nodal staging except in early-stage disease where the patient is a candidate for thoracoscopic lobectomy.
At thoracic exploration, the surgeon should assess not only those nodes attached to the resected specimen but also any abnormal nodes and by systematic biopsy of each node station. The current minimum standard is a systematic sampling of each lymph node station draining a tumor. For right-sided resections, nodes should be taken from mediastinal levels 2, 3, 4, 7, 8, and 9 as well as from the tracheobronchial angle and interlobar area (levels 10 and 11). On the left, the subaortic and anterior mediastinal nodes (levels 5 and 6) should undergo biopsy as well as levels 7, 8, and 9. Systematic sampling is required because of the frequent finding of pathologic N2 nodes involved in the presence of benign N1 levels (“skip” metastases), and even small, normal-appearing nodes harboring metastases, as demonstrated by Daly and colleagues.42 The incidence of unsuspected N2 disease after various staging pathways using modern imaging, including PET, and either routine or selective mediastinoscopy is at least 10%. Goldstraw and colleagues,88 for example, found pathologic N2 in 24% of clinical N0–1 cases. Further refinements of and experience with PET may decrease the gap between clinical and pathologic staging.
Some surgeons believe that complete mediastinal lymph node dissection (MLD) is indicated for diagnostic and therapeutic reasons in all resections for NSCLC. In the prospective randomized series reported by Sugi and associates272 and by Izbicki and colleagues,121 however, MLD did not increase overall survival. Operations following induction therapy for known N2 NSCLC should include MLD in virtually all cases. Although improved survival with routine MLD has not been proven, this approach does minimize sampling error by identifying N2 metastases that otherwise might have been missed, as shown by Graham and associates.91 In a subsequent report from the same group by Passlick and colleagues203 using immunohistochemical staining, no difference in survival was noted between the sampling and MLD patients overall or in those with micrometastases. However, in patients whose staining was negative, survival was superior in the MLD group. The results of the ACOSOG trial (Z0030) comparing lymph node sampling to MLD should be available sometime in 2010 or 2011.
Selection of the Operative Procedure
The appropriate operation depends on the clinical and surgical stage of the tumor and an accurate assessment of the structures involved. Lung cancers may be classified as occult, peripheral, or central. Occult lesions are not seen radiographically, but their presence is detected by sputum cytology or bronchoscopy. Central lesions are located radiographically within the central third of the hemithorax or bronchoscopically within or proximal to a segmental bronchus. Peripheral tumors are located beyond a segmental bronchus and in the outer two-thirds of the lung.
Occult Tumors
Most cases of occult NSCLC are brought to attention in screening programs for high-risk people or those who present with hemoptysis, cough, or wheezing. Because the lesion cannot be localized precisely anatomically, Cortese and colleagues39 had to perform a lobectomy 70% of the time and larger resections in the remaining patients. In a report of 94 cases by Saito and associates,249 resections included 58 lobectomies, 12 bilobectomies, 11 sleeve lobectomies, and 12 pneumonectomies.
In some patients, when occult NSCLC is confined to the bronchial mucosa or is an in situ carcinoma covering <3 cm of the mucosal surface, or in medically inoperable cases, photodynamic therapy (PDT) has been used successfully as primary treatment, as reported by Lam146 as well as by Kato,129 Cortese,40 and Weigel307 and their associates. Alternatively, brachytherapy can be delivered through bronchoscopically placed catheters, as reported by Taulelle and colleagues.279 Weigel and Martini306 reviewed these and other endobronchial approaches, such as laser, electrocautery, and cryoablation (see also Chapter 112). In most cases, however, the depth of invasion cannot be determined with certainty, and some have associated lymph node involvement.
Peripheral Tumors
The major considerations in the surgical evaluation of a peripheral tumor are its location within the lobe and its relation to other structures. Lesions that are clearly surrounded by parenchyma and confined to a single lobe are treated by lobectomy and occasionally by lesser resections. When a
peripheral tumor invades other structures, en bloc resection is often necessary. If a tumor abuts the chest wall on CT, the possibility of pleural invasion should be entertained, especially if the patient has associated pain. If the tumor approximates an interlobar fissure, the possibility of extension into an adjacent lobe should be considered. In all such cases, the operation should be planned and discussed with the patient by including the possibility of chest wall resection, bilobectomy, or pneumonectomy, as appropriate.
peripheral tumor invades other structures, en bloc resection is often necessary. If a tumor abuts the chest wall on CT, the possibility of pleural invasion should be entertained, especially if the patient has associated pain. If the tumor approximates an interlobar fissure, the possibility of extension into an adjacent lobe should be considered. In all such cases, the operation should be planned and discussed with the patient by including the possibility of chest wall resection, bilobectomy, or pneumonectomy, as appropriate.
Chest Wall
Tumors invading the chest wall are often resectable. The involved ribs should be transected several centimeters beyond the margin of gross involvement. In most cases, one rib and intercostal tissue above and below the tumor should also be included in the resection. Chest wall reconstruction is carried out as needed to prevent physiologic impairment due to paradoxical chest wall function or for cosmetic reasons (see Chapter 49). For posterior defects, support by the remaining chest wall muscles and scapula is usually sufficient, whereas anterior and lateral defects more often require reconstruction.
Although full-thickness resection is mandatory for tumors invading the osseous and muscular structures of the chest wall, there is controversy regarding the necessity of chest wall resection when invasion is confined to the parietal pleura. McCaughan and associates173 reported good results in such cases treated by the development of an extrapleural dissection plane when possible, stripping away the lung and parietal pleura from the endothoracic fascia, and proceeding to a full-thickness resection only if the margin was positive on frozen section. However, the experience of Trastek and colleagues283 suggests that chest wall resection is preferable even when invasion is confined to the parietal pleura. Ratto and associates221 stated that no attempt should be made to strip parietal pleura away and that an en bloc resection should be carried out when a tumor is firmly affixed to the parietal pleura. In support of this approach, Albertucci and associates7 found in 1992 that a histologic complete resection was achieved in only one-third of their patients treated by extrapleural dissection as compared with all of those undergoing a standard chest wall resection.
For isolated chest wall invasion with N0 or N1 positive nodes, there is no known role for neoadjuvant therapy. Trials for stage IIB and IIIA are under way, but whether this question can be answered from these trials is unclear, since the studies are not stratified for this type of tumor. Studies by Rusch242 and Kunitoh143 and their colleagues suggest that neoadjuvant chemoradiotherapy is beneficial in superior sulcus tumors but not specifically for isolated chest wall tumors. Superior sulcus tumors are dealt with separately in this chapter. Ginsberg and colleagues86 found no benefit for the use of brachytherapy in patients who had a complete resection of their chest wall tumors. They also found only a 9% five-year survival for the use of brachytherapy with or without preoperative or postoperative external beam radiation in patients who had an incomplete resection of their chest wall tumor. Similarly, adjuvant therapy after complete resection of a chest wall tumor is not indicated. Gould and colleagues90 from the Mayo Clinic reported in 1999 on their experience with 92 T3N0 patients. They found that patients with completely resected T3 N0 M0 NSCLC have similar local control and overall survival irrespective of primary location, type of surgery performed, or use of adjuvant radiation therapy.
Diaphragm
As noted by Rocco and associates236 and by Riquet and colleagues,233 the diaphragm is rarely involved by direct extension of NSCLC, despite the large area of contact between this structure and the base of the lung. When invasion occurs, that portion of the diaphragm should be resected with a wide margin of normal tissue without regard to the extent of the defect. Although unlikely to be helpful for NSCLC, it is feasible to resect and replace an entire hemidiaphragm. Unless the defect is small and can be closed primarily without tension, it should be replaced with a prosthetic material. Alternatively, a variety of muscle flaps can be used. When a large area of diaphragm has been resected or when the phrenic nerve has been resected, it is important that the diaphragm be reconstructed near the position of full inspiration to avoid paradoxical motion. When the defect is peripheral, it may be possible to reinsert the remaining cut edge at a higher level on the chest wall and thereby obviate the need for prosthetic material, as described by Daly.43
Pericardium
Total resection of the pericardium on the left can be performed without reconstruction. Partial defects should be closed to prevent herniation and strangulation of the left ventricle. On the right side, all pericardial defects, regardless of size, require repair. The potential problem if the pericardium remains open following right pneumonectomy is torsion of the heart into the hemithorax along the axis of the venae cavae, with consequent near total occlusion of venous inflow. Large defects can be closed with the pericardial fat pad, a pleural flap, or nonautologous material such as bovine pericardium or polytetrafluoroethylene (PTFE). Many surgeons suggest that a small opening be left in the repair or that the prosthetic material be fenestrated to prevent intrapericardial fluid accumulation and potential subsequent cardiac tamponade.
Vertebrae
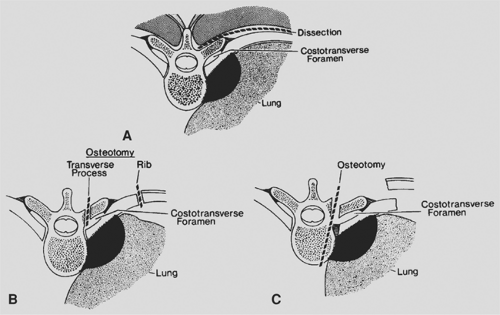
Tumors invading the vertebral bodies are rarely cured. Under most circumstances vertebral body invasion is considered T4 disease and thus unresectable. DeMeester and colleagues53 described a technique of partial vertebral resection for tumors fixed to the paravertebral fascia. They use a tangential osteotomy through the transverse process, costotransverse foramen, and superficial vertebral body (Fig. 110-1). The authors emphasize that this approach is not suitable for patients with radiographic evidence of bone destruction. Grunenwald and associates,94 however, reported on a small group of patients with radiographic evidence of osseous invasion treated by en bloc pulmonary resection and complete vertebrectomy with reconstruction by a combined anterior and posterior approach. In a subsequent report,95 this group had a 14% late survival in 19 patients treated by partial or total vertebrectomy. This technique should be limited to rare cases in which the tumor extent is completely delineated, node-negative, totally resectable, and, after careful evaluation with MRI, does not involve the spinal canal.
Superior Sulcus Tumors
Resection of peripheral tumors involving the apex of the chest and the lower portion of the brachial plexus (superior sulcus or Pancoast tumors) is discussed in Chapter 38. Although they
invade the chest wall, these challenging lesions are viewed as a distinct entity because of their unique clinical, anatomic, and surgical features. Since these tumors invade the first and/or second rib, the traditional margin easily obtained on lower chest wall lesions is impossible. Therefore, when a superior sulcus tumor is deemed potentially resectable at presentation, operation is preceded by induction therapy with chemotherapy and radiation therapy. Two trials recently completed show that there is a better response rate, resection rate, and an extended survival. Rusch and associates245 reported in 2007 on the ECOG 9416 (Intergroup Trial 0160) and Kunitoh and colleagues143 reported in 2008 on the Japan Clinical Oncology Group Trial 9806. The ECOG trial had a 76% complete resection rate with a 44% 5-year survival. The Japanese trial had a 68% complete resection rate and a 56% 5-year survival.
invade the chest wall, these challenging lesions are viewed as a distinct entity because of their unique clinical, anatomic, and surgical features. Since these tumors invade the first and/or second rib, the traditional margin easily obtained on lower chest wall lesions is impossible. Therefore, when a superior sulcus tumor is deemed potentially resectable at presentation, operation is preceded by induction therapy with chemotherapy and radiation therapy. Two trials recently completed show that there is a better response rate, resection rate, and an extended survival. Rusch and associates245 reported in 2007 on the ECOG 9416 (Intergroup Trial 0160) and Kunitoh and colleagues143 reported in 2008 on the Japan Clinical Oncology Group Trial 9806. The ECOG trial had a 76% complete resection rate with a 44% 5-year survival. The Japanese trial had a 68% complete resection rate and a 56% 5-year survival.
Resection involves removal of the involved portions of the apical chest wall—typically including the first, second, and third ribs and the T1 nerve root—along with lobectomy. A lesser pulmonary resection is now considered inadequate by most surgeons. Invasion of the vertebral body or of the subclavian vessels and clinical N2 disease generally contraindicate primary operation and a more aggressive approach, especially after induction therapy, should remain the subject of clinical investigation. These tumors may be resected through either a high posterolateral thoracotomy or an anterior approach using a cervical incision with extension down to the second intercostal space and resection of the first and second costal cartilages. Darte- velle and colleagues50 have used the anterior approach alone to perform extensive vascular resection along with lobectomy in this setting. It is important to bear in mind that these tumors are in an advanced stage; thus many patients do not reach the surgical option. Kappers and colleagues127 reviewed their experience in the Netherlands in 2008. They found that 38% of their patients did not complete therapy for a variety of reasons, including progression to stage IV disease, comorbidity, unresectability (extensive tumor growth and/or persisting N2–3 status) or insufficient response to induction treatment.
Central Tumors
Central tumors are more likely to be associated with malignant lymphadenopathy and to involve mediastinal structures. Accor- dingly, careful imaging and invasive evaluation are mandatory before consideration of thoracotomy. By definition, complete resection requires at least a lobectomy and often requires a pneumonectomy or more extended procedure. Central bronchial T3 lesions and some T4 cancers involving the carina can be treated successfully by primary operation, in the latter situation usually by a carinal pneumonectomy. The selection, techniques, and results of resection in this setting are discussed in Chapters 29 and 31. The decreasing mortality rate associated with these extensive procedures has been noted previously.
Infrequently, for a small lesion in the left mainstem bronchus, a localized bronchial sleeve resection with pulmonary conservation can be carried out, as reported by Cerfolio and Bryant36 in 2007. Localized tumors involving the pulmonary veins (even with extension into the pericardium and left atrium) may be amenable to resection by excision of a contiguous cuff of the atrium, using vascular clamps and sutured closure or vascular staplers.
Central NSCLC with local invasion limited to the mediastinal pleura and adipose tissue but not involving deeper structures is also often suitable for total resection. With exceedingly rare exceptions, in contrast, tumors invading the superior vena cava or the aorta or its branches should not be addressed by primary operation but rather considered for resection in a few cases only after postinduction reassessment. It cannot be overemphasized that in all cases of locally invasive NSCLC considered for resection, the absence of N2 lymphadenopathy should be confirmed by rigorous preoperative staging.
Simultaneous Cardiac Operation and Pulmonary Resection
When a patient requires myocardial revascularization or any other cardiac procedure and also has a resectable lung cancer, the question of simultaneous versus staged procedures arises. This clinical situation can arise during the physiologic assessment of a lung cancer patient being considered for resection or in the preoperative radiographic evaluation of a cardiac patient. When the lung cancer can be resected through a median sternotomy, the timing of the procedures is largely a matter of the surgeon’s preference and patient-specific factors.
The experiences reported by Terzi281 and Danton47 and their colleagues support the safety and efficacy of simultaneous operations. The cardiac procedure should be performed first, without complications. Pulmonary resection is carried out after reversal of anticoagulation and confirmation of hemodynamic and hemostatic stability. Generally a lobectomy is carried out, although pneumonectomy has been reported by Piehler210 and Danton47 and their associates. Because cardiac retraction required during transsternal left lower lobectomy may cause hemodynamic problems, most left-lower-lobe tumors should be resected at a separate session. Limited resections should be used only with the appropriate indications. In all cases except emergent cardiac surgery, a full staging evaluation of the lung tumor should be carried out before operation.
Combined procedures have the advantage of a single operation and recovery as well as absence of delay in cancer treatment. However, concerns have been raised about the adverse oncologic effect of immunosuppression and the possibility of tumor dissemination associated with cardiopulmonary bypass if the cardiac operation is performed before or concurrently with the pulmonary resection. This might be answered by comparing on-pump and off-pump procedures combined with lung resection. Schoenmakers and associates257 in 2007 made this comparison in 43 patients: 28 had lung resection followed by coronary artery revascularization on-pump and 14 had off-pump coronary artery bypass first followed by lung resection. No difference in survival between groups was found. Subsequently however, in 2008, Dyszkiewicz et al.64 reported their experience with 25 patients having off-pump coronary bypass procedures and an associated resection for lesions ranging equally between IA and IIIA disease. Although they report in the abstract that 68% survived during the observation period, the Kaplan–Meier curve tells a different story. The median survival was 30 months, with zero probability to survive as long as 5 years. All of their patients died of recurrent disease.
Synchronous Lung Cancers
Although the incidence of a second primary lung cancer in patients previously treated for NSCLC is more than 10%, as discussed by Ponn214 in 2000, the prevalence of synchronous primary lung cancers has risen. The nature of multiple primary sites of involvement appears to have changed coincident with the rise in the proportion of adenocarcinomas among NSCLC patients. Multiple sites of parenchymal adenocarcinoma at presentation are now more commonly encountered than multiple sites of squamous cancer in the airways. Nakata and colleagues188 in 2007 reviewed their experience with resected adenocarcinoma between 1994 and 2002. They noted an incidence of 8.4% of patients with synchronous primaries. Both the diagnosis of synchronous lesions, as opposed to metastases, and the optimal surgical and nonsurgical treatment of such lesions represent challenges.
Criteria for differentiating between synchronous primary and metastatic lesions established by Martini and Melamed163 have traditionally been accepted by most surgeons. For lesions to be considered synchronous primaries, they must be physically distinct and separate. The histology must be different or, if similar, the neoplasms must have an origin from carcinoma in situ or be located in different pulmonary segments and have no carcinoma in the lymphatic vessels and nodes common to both lesions and no extrapulmonary metastasis. In 1995, Antakli and associates12 modified the criteria for tumors of similar histology, regarding them as separate primaries if two or more of the following five conditions were met: (a) anatomically distinct, (b) presence of associated premalignant lesions, (c) absence of systemic metastasis, (d) no mediastinal disease, and (e) different DNA ploidy. More recently, in 2006, Su and associates270 described the use of three immunohistochemical markers: thyroid transcription factor-1 (TTF-1), cytokeratin 7 (CK7), and cytokeratin 20 (CK20) to distinguish between primary lung and metastatic disease. They noted that TTF-1 is present in lung primaries 73% of the time. CK7 expression was significantly more frequent in adenocarcinomas of pulmonary and breast origin than gastrointestinal (GI) origin, and CK20 expression was significantly more prevalent in adenocarcinoma that originated in the GI tract. Thus, the use of these three markers significantly increased the accuracy of defining the origin of adenocarcinomas in the lung. In all likelihood, use of immunohistochemistry will supplant all other methods of recognizing primary lung cancers.
In general, anatomic resection should be carried out, especially for the larger or more central tumor. The specifics of each case will dictate the surgical approach, from sternotomy to bilateral thoracoscopy or thoracotomy to staged operative procedures. It may be best to stage each side in order to allow time for the pathologist to perform the required immunohistochemistry. The more difficult and as yet unresolved question is whether or not to perform a pneumonectomy for two lesions in different lobes, both of low-grade potential, such as bronchioloalveolar carcinoma without an invasive component. Similar guidelines should be followed when dealing with metachronous NSCLC, although surgical options for new cancers following prior pneumonectomy may be limited.
Incomplete Resection
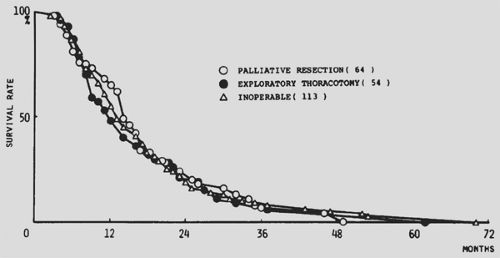
Incomplete resection of NSCLC is variously defined, including macroscopic disease left behind, microscopic positive margins, involvement of the highest resected mediastinal lymph node, and the presence of remaining intrapulmonary or distant metastases. Grossly incomplete resection of NSCLC (R2) does not result in long-term survival. With current staging methods, grossly complete resection should be achieved in 99% or more of operated patients. Except under very unusual circumstances—such as infection, bleeding, or pain that cannot be palliated by other means—intentional incomplete resection should not be considered. As shown early on by Hara and associates,98 patients with advanced NSCLC who have an incomplete resection (whether of the primary tumor or metastatic lymph nodes) have a clinical course identical to those who undergo nonresective exploration or no operation (Fig. 110-2). Furthermore, patients who undergo incomplete resection may have a poorer quality of remaining life than those treated by other modalities or by supportive care.
Kimura and Yamaguchi133 found a range of 5-year survival when 279 incomplete resections were classified according to the site of residual cancer. When incomplete resection involved the chest wall, survival was 29%, versus only 5% for residual disease in lymph nodes. Others have found lower survival for incompletely resected chest wall cases. Lacasse and coworkers145 of the
Canadian Lung Oncology Group found that histologic positivity of surgical margins or of the highest resected lymph node did not predict clinical recurrence, but follow-up was only 3 years. This is the only report that questions the negative prognostication of an incomplete resection.
Canadian Lung Oncology Group found that histologic positivity of surgical margins or of the highest resected lymph node did not predict clinical recurrence, but follow-up was only 3 years. This is the only report that questions the negative prognostication of an incomplete resection.
Residual tumor at the bronchial resection margin also has prognostic value. Shields259 described three categories of positive bronchial margins: (a) gross disease, (b) microscopic tumor in the peribronchial tissue, and (c) microscopic residual cancer at the mucosal or submucosal margin. Although the first two findings portend a poor prognosis, late survival without adjuvant therapy occurred in about one-fourth of patients in the third group. Similar findings were reported by Soorae and Stevenson.264 More recently, however, Gebitekin and associates83 noted that a microscopically positive bronchial or peribronchial margin adversely affected survival in N2 NSCLC, no survival versus 17% 5-year survival for N2 with a clear margin. Snijder and associates263 found that for stage I NSCLC, either a peribronchial or mucosal margin positive for invasive cancer was associated with late survival of 27%, versus 58% for carcinoma in situ or a negative margin. Massard and colleagues171 reported a 55% 5-year survival rate for in situ carcinoma, as compared with 20% for microscopic invasive mucosal or peribronchial residual cancer. Ghiribelli and coworkers84 likewise found no difference in the negative impact on survival between mucosal and extramucosal involvement. Passlick and associates202 emphasized the importance of lymphatic invasion as a negative factor regardless of the bronchial or extrabronchial location of positivity.
Because of these findings, intraoperative frozen section assessment of the airway margin should be employed frequently and a more proximal resection carried out if indicated. When an involved margin is detected only on final pathologic review (especially when lymphatic invasion and lymph node disease are absent), reoperation to achieve more proximal airway resection should be considered, as suggested by Kaiser,126 Liewald,148 Snijder,263 and Ghiribelli84 and their associates. Many patients, however, will not be candidates for this aggressive approach. Although external radiation therapy has not been shown to prolong survival in the overall spectrum of incompletely resected NSCLC, adjuvant radiation therapy should be considered because local control may be enhanced. The role of brachytherapy is also unclear because there are no randomized studies and experience is limited. Although the risk associated with this method—when isotopes are implanted in tissues such as the chest wall—is minimal, its use in grossly normal bronchial margins and other vital structures for microscopic positivity may result in necrosis and its sequelae.
The International Association for the Study of Lung Cancer in conjunction with the International Union Against Cancer (UICC), reported by Rami-Porta219 in 2005, proposed definitions of resection margins to be included in the next staging update. Complete resection requires all of the following: free resection margins proved microscopically; systematic nodal dissection or lobe-specific systematic nodal dissection; no extracapsular nodal extension of the tumor; and the highest mediastinal node removed must be negative. Whenever there is involvement of resection margins, extracapsular nodal extension, residual positive lymph nodes, or positive pleural or pericardial effusions, the resection is defined as incomplete. When the resection margins are free and no residual tumor is left but the resection does not fulfill the criteria for complete resection, there is carcinoma in situ at the bronchial margin or positive pleural lavage cytol- ogy, the term uncertain resection is proposed.
Results of Surgical Treatment
Surgical results are judged by the same standards as other modalities in terms of long-term survival and disease-free interval. Chemotherapy and radiation therapy take place over relatively prolonged time periods with symptoms and toxicities developing over hours to days and resolving over days to weeks. In contradistinction, surgical results begin at the operating room door and can be defined in the number of minutes in the operating room. The consequences of operation are immediate and are dependent on the acute stress response to the operation, immediate and intermediate perioperative complications, postoperative length of stay, days and weeks of recovery and rehabilitation, and short- and long-term incisional pain syndromes. The acute risk is dependent mainly on patient-specific factors, such as age, gender, race, comorbidities and prior (neoadjuvant) therapy. Tumor-related factors are important insofar as they dictate the extent of resection.
Long-term survival is determined by multiple factors. At present, the most valuable predictors are the tumor stage and
the tumor node metastasis (TNM) subsets within each stage. As emphasized, the ability to accomplish a complete resection is also paramount and largely depends on the tumor stage. In assessing surgical results, the concept of clinical stage versus pathologic stage must be clearly appreciated (see Chapter 109). The clinical stage is based on a synthesis of all invasive and noninvasive studies short of resection. The pathologic stage is determined from the resected tissue and may or may not coincide with the clinical stage. Surgical decisions are made based on the clinical stage, but most surgical reporting is based on the pathologic stage. In 2005, Gal and colleagues79 authored for the College of American Pathologists an update on their reporting checklist, which includes cell type; tumor dimensions; tumor grade; invasion into visceral pleura, large veins, lymphatics; surgical margins; number of positive nodes and total number by station; and other tumors. In the age of routine CT and PET scanning, fewer cases have disparate clinical and pathologic staging. In a study on the additive value of mediastinoscopy to CT and PET, Meyers and associates176 noted that only 3% of patients had an upstaging by mediastinoscopy. More importantly, in patients with stage IA lung cancer, the total difference between clinical staging and postresection pathologic staging was 5.6%. Lee and colleagues148 in 2007 noted that central tumors >2 cm had a high potential for radiologically occult N2 metastasis. They also noted that if the primary tumor had an SUV >4, 10% had radiologically occult metastasis. However, most were discovered with mediastinoscopy. Thus there should be very close correlation between CT, PET, and mediastinoscopy preresection clinical staging and final postresection pathologic staging.
the tumor node metastasis (TNM) subsets within each stage. As emphasized, the ability to accomplish a complete resection is also paramount and largely depends on the tumor stage. In assessing surgical results, the concept of clinical stage versus pathologic stage must be clearly appreciated (see Chapter 109). The clinical stage is based on a synthesis of all invasive and noninvasive studies short of resection. The pathologic stage is determined from the resected tissue and may or may not coincide with the clinical stage. Surgical decisions are made based on the clinical stage, but most surgical reporting is based on the pathologic stage. In 2005, Gal and colleagues79 authored for the College of American Pathologists an update on their reporting checklist, which includes cell type; tumor dimensions; tumor grade; invasion into visceral pleura, large veins, lymphatics; surgical margins; number of positive nodes and total number by station; and other tumors. In the age of routine CT and PET scanning, fewer cases have disparate clinical and pathologic staging. In a study on the additive value of mediastinoscopy to CT and PET, Meyers and associates176 noted that only 3% of patients had an upstaging by mediastinoscopy. More importantly, in patients with stage IA lung cancer, the total difference between clinical staging and postresection pathologic staging was 5.6%. Lee and colleagues148 in 2007 noted that central tumors >2 cm had a high potential for radiologically occult N2 metastasis. They also noted that if the primary tumor had an SUV >4, 10% had radiologically occult metastasis. However, most were discovered with mediastinoscopy. Thus there should be very close correlation between CT, PET, and mediastinoscopy preresection clinical staging and final postresection pathologic staging.
The vast literature on the surgical treatment of NSCLC is confusing. A few general principles for assessing individual reports may facilitate comparison. Of obvious importance is the time period encompassed, not only because of variations in imaging but also because of evolution of surgical techniques and philosophy and clinical staging methods. Of utmost importance is recognition of the subset of patients on which the conclusions are based (whether the entire group, all operated patients, or only those who had a complete resection). Many authors use the diminishing denominator to present more optimistic results. Perspective may be enhanced by noting the actual number of long-term survivors with respect to the initial number of cases, in addition to the actuarial probability of survival. Although the latter is a valid figure for statistical reporting, the methodology is such that a numerically small surviving cohort may yield a surprisingly favorable actuarial probability of survival.
Another variable requiring attention is the use of preoperative and postoperative adjuvant treatments that, in many surgical reports, are not factored into the conclusions. Similarly, it is very rare for surgical reports to include all patients who present with a given stage, especially when advanced, but rather only those selected for operation. Before sweeping conclusions can be drawn, analysts must view the presented experience in the overall spectrum of lung cancer.
This discussion focuses on surgical results based on TNM staging and simple anatomic factors. Late results are presented as 5-year actuarial survival unless otherwise specified. Although the current staging system by Mountain182 will be replaced in the future by the seventh edition, the material is presented according to the currently used system. Table 110-3 presents survival by stage and TNM subsets from the reports of Mountain,182 van Rens and colleagues,289 Naruke and associates,190 and Asamura et al.14 These four series were selected as representative because of their large numbers of patients, their span of nearly three decades and three continents, and their presentation of the material in a similar format.
Many other valuable reports are noted in the text and tables of this chapter. Reports that are unclear or present the material in a nonstandard fashion, without a cogent alternative point, are not included. For now, the recommendations for changing the staging system are under consideration. Because the recommendations radically change some stages and make it impossible to compare groups, no attempt is made to include the proposed changes in a discussion of results by stage.
Table 110-3 Five-Year Survival by TNM Classification and by Stage According to the 1997 System | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Occult Non-Small-Cell Lung Cancer
Truly occult lung cancer discovered incidentally in an asymptomatic patient with no radiographic abnormality is exceedingly rare. Most series of resections of these tumors predate the ubiquitous use of CT scans and flexible bronchoscopes with digital processors. Those patients with tumors that are truly occult who are fortunate to have a complete resection have a very high cure rate.
Incidentally Discovered Non-Small-Cell Lung Cancer
Although incidental NSCLC does not constitute a specific stage, many of the least advanced tumors are detected in this manner, and the results of resection in this group are excellent. These tumors may present in two ways. The tumor may not be apparent on radiographic studies and present with symptoms such as persistent cough or hemoptysis. Alternatively, the lesion may be discovered on radiographic screening studies.
Most radiographically occult cancers are squamous cell lesions, and many involve the large airways. Saito and colleagues249 reported 94 patients with occult squamous lung cancer who were treated by resection. Although all were clinical stage I, pathologic staging showed 16 TisN0 (stage 0), 72 T1N0 (stage IA), and 6 stage IIA and B (4 T1N1 and 2 T2N1). The 5-year actuarial survival rate overall was 80%. Watanabe and coworkers303 treated 20 patients with occult central cancers, mainly by bronchoplastic resections, and noted a 10-year survival of 92%. Among 98 cases of centrally located occult cancers reported by Koike and associates,137 most underwent lobectomy or bilobectomy, pathologic stages included 5 TxN0, 19TisN0, 63 T1N0, and 11 T2N0, and the late survival rate was 81% overall and 89% for in situ lesions. Sagawa and colleagues248 performed segmentectomy for suitable, more peripheral radiographically occult cancers in 58 patients. The 5-year survival rate in this series was 83% overall. Most reports, however, note a disturbing incidence of synchronous and metachronous multiple aerodigestive squamous cancers in this patient population. Saito and associates249 noted an incidence of 9.6% synchronous and 7.4% metachronous lesions.
Screening of large populations of at-risk patients has generated a new conundrum. Many lesions discovered at screening are small, peripheral ground-glass lesions that turn out to be noninvasive bronchioloalveolar cancers. A number of these patients have more than two similar lesions in disparate parts of the lungs. Raz and associates222 noted in 2007 that 36% of their patients over 5 years had incidental cancers detected on screening. These lesions were mostly noninvasive bronchioloalveolar cancers. The patients had curative resections, but the study was not powered to determine whether there was a difference in survival compared with patients who had symptomatic tumors. Earlier, in 2005, Ashton and Jett13 made the observation that “Although there are promising data on the sensitivity of these newer screening methods, especially low-dose CT, for detecting early lung cancer, none of the published trials are controlled, and they have not yet proven a decrease in mortality.” They issued a strong caution that any patient desiring screening should be part of a controlled trial to attempt to answer the role of screening and early ablation or resection of low-grade malignancies.
Stage I: Localized Node-Negative Non-Small-Cell Lung Cancer
Tumors of relatively small size that are contained within a single lobe without lymph node metastasis defines the stage of non-small-cell cancer that should have the best prognosis. When the cancer is <3 cm, it fits the definition of a T1N0M0 stage IA tumor. From a surgical standpoint, simple lobectomy with lymph node sampling or lymphadenectomy is the operation that produces an R0 resection. It is akin to removing a diseased bush from a hedge. It completely removes the disease along with the roots, so as to prevent its spread to other bushes within the hedge. Lesions >3 cm but that otherwise fit the definition above are classified as T2 tumors. Lesions of this size often extend out of the zone of one lobe. Other descriptors have been added to this T stage—such as visceral pleural involvement, location in a main bronchus >2 cm from the carina, or causing bronchial obstruction—simply because tumors of this size often have these characteristics. Theoretically, they have a greater chance for local spread and for metastasis. Surgically, T2 tumors may require more than a single lobectomy to obtain a complete resection. These lesions are classified as T2N0M0 stage IB cancers. Yet complete resection should lead to a good long-term result. The 1997 staging system recognized these facts and considered these tumors in the best prognostic category.
Postsurgical 5-year survival rates in the T1N0 group (stage IA) are uniformly excellent, whereas postresection survival in IB disease is less satisfactory. In both categories, however, the efficacy of resection is currently not matched by any other treatment modality. In addition, there is at present no evidence that additional therapy as induction or adjuvant offers benefit over resection alone. Although clinical trials are in progress, Pignon,211 reporting in 2008 for the Lung Adjuvant Cisplatin Evaluation Collaborative, noted that adjuvant chemotherapy containing cisplatin actually had a negative effect on survival for both stage IA and IB.
In the databases of Mountain182 and those of van Rens,289 Naruke,190 and Asamura14 and their colleagues, 5-year postsurgical survival rates for pathologic stage IA cases are 67%, 63%, 75%, and 84% respectively. Many surgeons worldwide have confirmed these results, and some have reported higher survival rates in cooperative and single-center series spanning three decades (Table 110-4). The range of reported late survival is from 63% to 85% for stage IA and 43% to 78% for stage IB.
Influence of Size on Survival
Even in cases of limited stage I node-negative NSCLC, additional factors may influence survival, including tumor size, cell type, and central versus peripheral location. Among these variables, the size of the primary lesion most consistently has been found to influence prognosis. Read and associates224,225 noted significantly better survival for T1N0 tumors ≤2 cm than for T1 lesions between 2 and 3 cm. Similarly, Ishida and coworkers117 found a more favorable prognosis for tumors <1 cm compared with those 2 to 3 cm but no difference between T1N0 cancers ≤1 cm and tumors 1.1 to 2 cm. Padilla and colleagues199 reported markedly better late survival for smaller-diameter pT1N0 tumors (87% at 5 years and 74% at 10 years for ≤2 cm versus 65% at 5 years and 49% at 10 years for cancers 2.1–3 cm). In the
provocative report of the IASLC group studying T stage for possible revision, Rami-Porta et al.219 noted that there was a breakpoint in survival using log-rank analysis between T1N0 tumors <2 cm and tumors 2 to 3 cm in size (Fig. 110-3). They also found a break in the T2 tumors 3 to 5 cm and 5 to 7 cm in size independent of other qualifiers for T2, such as bronchial obstruction and visceral pleural involvement. The 5-year survival among the 7,116 node-negative T1 and T2 patients divided into these groups was 77%, 71%, 58%, and 49%, respectively.
provocative report of the IASLC group studying T stage for possible revision, Rami-Porta et al.219 noted that there was a breakpoint in survival using log-rank analysis between T1N0 tumors <2 cm and tumors 2 to 3 cm in size (Fig. 110-3). They also found a break in the T2 tumors 3 to 5 cm and 5 to 7 cm in size independent of other qualifiers for T2, such as bronchial obstruction and visceral pleural involvement. The 5-year survival among the 7,116 node-negative T1 and T2 patients divided into these groups was 77%, 71%, 58%, and 49%, respectively.
Table 110-4 Selected Series Reporting Postresection 5-Year Survival Rates for Pathologic Stage IA (T1N0) and Stage IB (T2N0) Non-Small-Cell Lung Cancer | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree