CHAPTER 36 Surgical Treatment of Benign Esophageal Diseases
THE ESOPHAGUS AND ITS SURROUNDINGS
Esophageal Wall
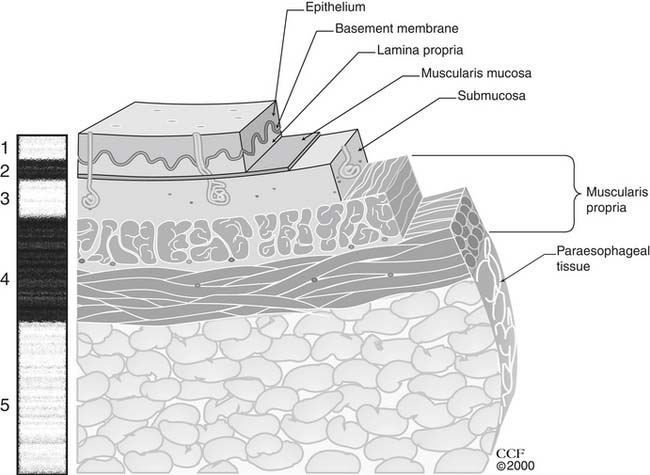
The esophagus is lined with stratified, nonkeratinizing squamous epithelium (Fig. 36-1), isolated from the remainder of the esophageal wall by a basement membrane. Immediately beneath the basement membrane is the lamina propria, a thin layer of loose connective tissue with a complex of collagen and elastic fibers. It contains a network of endothelium-lined channels, both capillaries and lymphatics. The muscularis mucosae supports the lamina propria and is composed of a longitudinal layer of smooth muscle. This continuous muscle layer pleats the inner layers of the esophagus into a series of folds that disappear with distension. The epithelium, lamina propria, and muscularis mucosae comprise the esophageal mucosa.
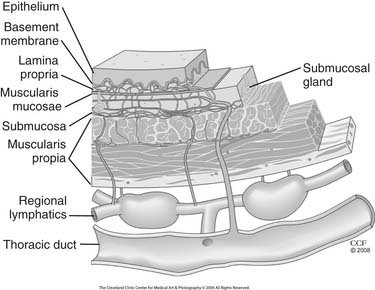
Figure 36–1 Esophageal wall and its unique lymphatic drainage.
(The Cleveland Clinic Center for Medical Art and Photography. © 2009. All rights reserved.)
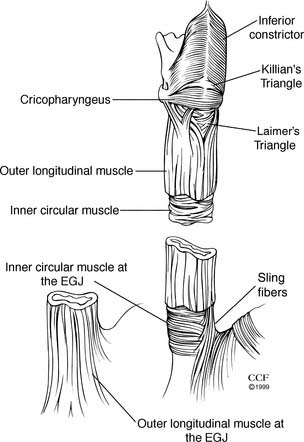
The muscularis propria is the muscular sleeve that provides the propulsive force necessary for swallowing. There are two layers of muscle: an inner circular layer and an outer longitudinal layer. The proximal 4% to 5% of the esophagus is composed completely of striated muscle and the distal 54% to 62% completely of smooth muscle.1 Smooth muscle first appears in the anterior circular layer. The transition from striated to smooth muscle in the circular muscle layer is gradual, and the 50% point is approximately 5 cm from the cricopharyngeus muscle.
The cricopharyngeus (upper esophageal sphincter [UES]) is a continuous transverse band of muscle originating from the cricoid cartilage (Fig. 36-2). Superiorly, the muscle of the cricopharyngeus blends with the inferior pharyngeal constrictor muscle. A posterior defect, Killian’s triangle, is an inverted fan–shaped weakness in the inferior constrictor at the superior border of the cricopharyngeus. Inferiorly, the cricopharyngeus merges with the inner, circular layer of the muscularis propria. The longitudinal muscle layer of the muscularis propria originates from the lateral aspect of the cricoesophageal tendon. Posteriorly, these anterior and lateral components converge to meet at the midline. Thus, the proximal 1 to 2 cm of the posterior cervical esophagus is composed only of inner circular muscle, creating a potential for a mirror-image triangular area of weakness called Laimer’s triangle.
Contraction of the longitudinal muscle fibers of the esophageal body produces esophageal shortening. The inner circular muscle is arranged in incomplete rings producing a helical pattern. Muscle layers are equal and uniform in thickness until the distal 3 to 4 cm of the esophagus. Here, the inner circular layer thickens and divides into incomplete horizontal muscular clasps on the lesser curve aspect of the distal esophagus, and oblique fibers on the greater curve aspect. These become gastric sling fibers (see Fig. 36-2). Although no complete circular bands exist at the lower esophageal sphincter (LES), it is this area of rearranged circular fibers that corresponds to the high-pressure zone of the LES.
Lymphatics begin as blind endothelium-lined saccules in the lamina propria just below the epithelium and basement membrane. Recent immunohistochemical study of the esophageal wall using lymphatic endothelial marker D2-40 has provided new insight into the lymphatic anatomy of the esophageal wall (see Fig. 36-1).2 There is a dense longitudinal plexus of lymphatic vessels in the lamina propria. Rare perforating lymphatics have been found draining into a sparse circumferential lymphatic network in the outer margin of the submucosa. Perforating lymphatics from the submucosal plexus, usually running with an artery and vein, penetrate the inner circular layer of the muscularis propria. Here, they drain into a circumferential intramuscular plexus that accompanies the artery, vein, and nerves of this space. Afferent lymphatics usually accompanied by an artery and a vein drain the intramuscular plexus into the lymphatic channels in the adventitia. No direct connection from the lamina propria network and the thoracic duct has been identified.2 Existence of direct routes from mural lymphatics to the thoracic duct, without a relay through regional lymphatics and lymph nodes, has been documented by many authors; however, the exact patterns and occurrence of these pathways are highly variable.3–5
The arterial supply of the esophagus is parasitic. It is derived from blood vessels supplying other organs in the neck, chest, and abdomen. Generally, these vessels divide at a distance from the esophagus and send small segmental branches to that segment of the esophagus. Esophageal blood supply has three principal sources. The superior and inferior thyroid arteries supply the cervical esophagus. The proximal and middle thoracic esophagus receives blood from branches of the bronchial arteries. The only dedicated esophageal arteries are one or two branches that arise from the anterior aspect of the aorta below the tracheal carina. In a third of autopsy specimens, no esophageal artery could be identified.6 These esophageal arteries directly supply the lower thoracic esophagus. The lower thoracic esophagus and abdominal esophagus receive arterial branches from the left gastric and, occasionally, the splenic arteries. The combination of a segmented arterial supply derived from multiple sources and a rich intramural vascular plexus ensures an excellent esophageal blood flow and permits extensive esophageal mobilization without esophageal arterial insufficiency or ischemia. Because esophageal arteries branch from larger arteries some distance from the esophagus, stripping of the esophagus from its bed during transhiatal (blunt) esophagectomy is possible without direct ligation of the esophageal arterial supply. Arterial spasm provides adequate hemostasis; thus, significant bleeding does not complicate this procedure.
Subepithelial esophageal venules drain into a substantial submucosal venous plexus that extends the length of the esophagus.7 There are venous connections between the lower thoracic and abdominal esophagus and the portal venous system. Venules then pierce the muscularis propria to drain into veins on the surface of the esophagus. Regional drainage is directed to the inferior thyroid and brachiocephalic veins in the neck, the azygos and hemiazygos veins in the chest, and the left gastric and splenic veins in the abdomen.
Regional Anatomy
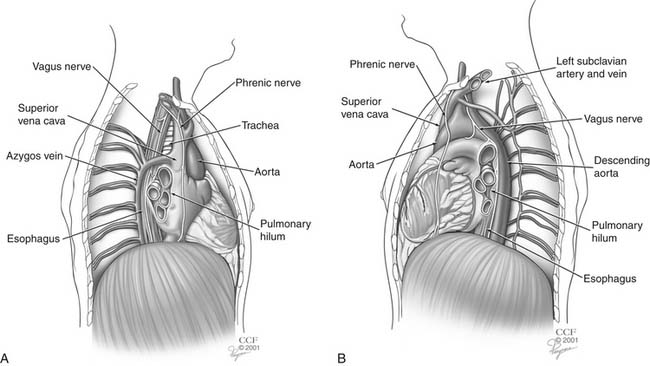
The esophagus spans the lower neck, thoracic cavity, and upper abdomen (Fig. 36-3). The anatomy of the esophagus is best divided into fifths: cervical, upper thoracic, middle thoracic, lower thoracic, and abdominal esophagus. The anterior wall of the cervical esophagus is in intimate contact with the posterior membranous trachea. The recurrent laryngeal nerves course anteriorly and laterally in the tracheoesophageal groove. The carotid sheaths bind the cervical esophagus laterally. The posterior wall of the cervical esophagus lies on the vertebral bodies.
EVALUATION OF THE ESOPHAGUS
History and Physical Examination
Box 36-1 lists systemic diseases with esophageal manifestations. Some are discussed later in this chapter. These potential underlying disorders should be considered while obtaining the patient’s history and performing the physical examination.
Investigations
Barium Esophagram
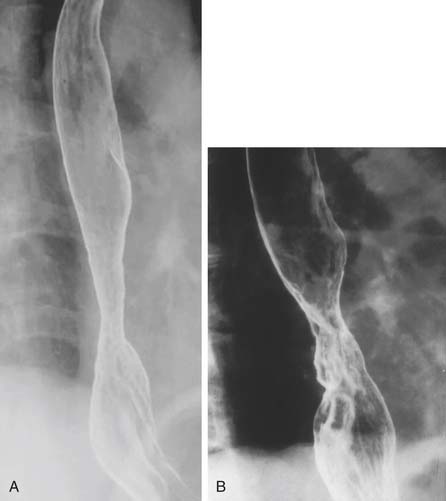
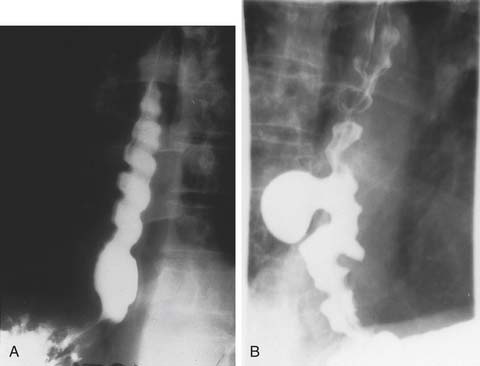
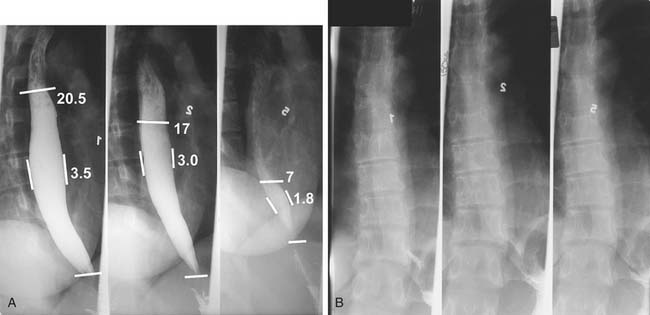
A three-phase study assessing mucosa, contour, and function of the esophagus is optimal.8 First, the mucosa is examined in the double-contrast phase, in which the patient, in the upright position, ingests high-density barium and CO2 tablets (Fig. 36-4). Next, esophageal function is assessed with the patient in the right anterior oblique (RAO) position and ingesting low-density barium in single swallows at 20- to 30-second intervals (Fig. 36-5). The examination is videotaped. The value of attempting to elicit reflux in this phase is questionable, because 20% of normal individuals have radiologic reflux.9 Barium tablets, or barium-coated marshmallows or solids, may demonstrate abnormalities not visualized by liquid barium studies. The final phase, the full-column technique, is performed with the patient in a semiprone RAO position and with low-density barium. Multiple quick swallows produce a column of barium that fully distends the esophagus. This optimizes imaging of the distal esophagus and can demonstrate small hiatal hernias, subtle strictures, or distal rings (Fig. 36-6). The esophagus is allowed to empty, and the remaining barium coating the esophageal wall provides a mucosal relief study, now rarely performed.
A timed barium esophagogram is a simple test of esophageal emptying (Fig. 36-7). After ingestion of a premeasured amount of barium, usually 250 mL, spot films are taken at 1-, 2- and 5-minute intervals and, if necessary, at 10 minutes and 20 minutes after barium ingestion. This allows simple quantification of esophageal emptying and is useful for evaluating motility disorders and to follow therapy.10,11
Esophageal Manometry
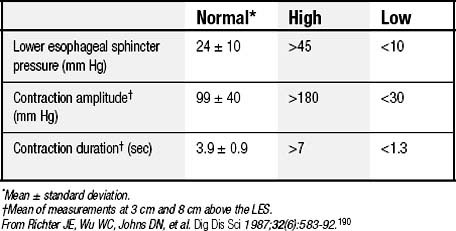
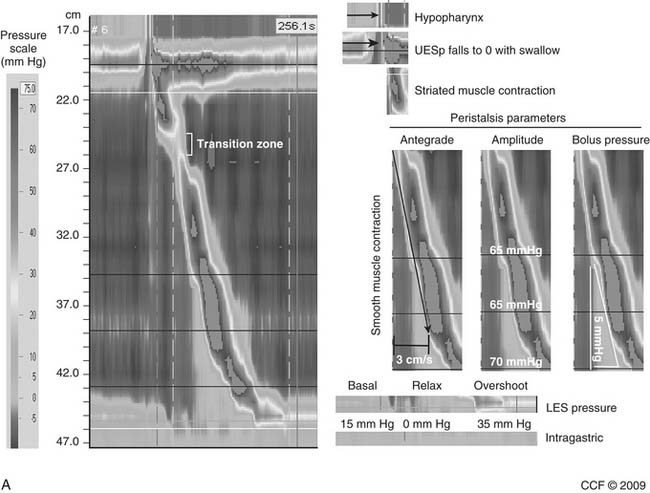
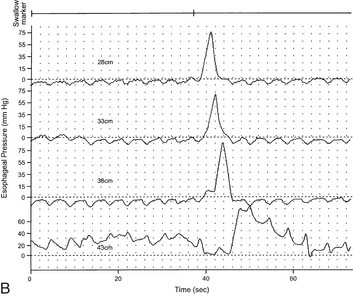
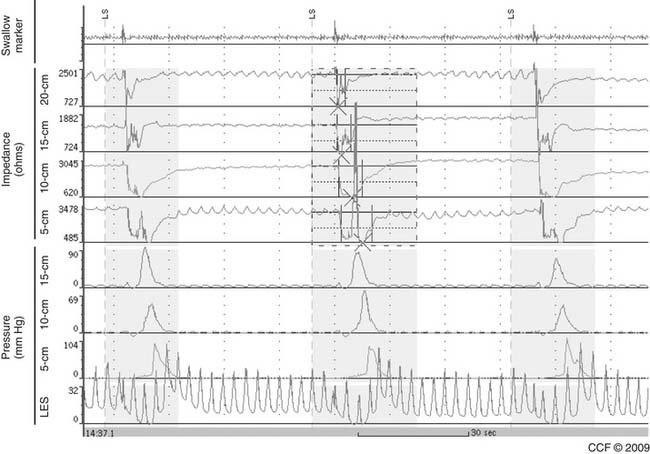
LES resting pressure and length, but most importantly completeness of LES relaxation, are measured (Table 36-1). HRM allows more accurate measurement of LES relaxation than traditional manometry by compensating for the LES movement with swallows. Esophageal body parameters to assess are antegrade esophageal peristalsis, contraction amplitude, and duration. HRM allows generation of a spatiotemporal plot, where segments of the peristaltic wave-front can be separately analyzed (Fig. 36-8).12 Direct comparison with traditional manometry shows that HRM is more accurate at predicting abnormal barium bolus transport, especially with mild or moderate esophageal dysmotility.13 Reclassification of esophageal motility disorders based on HRM has been suggested,12,14 but further reports, including outcome studies, are needed to confirm validity. UES measurements have not been found to be clinically useful.
Ambulatory Conventional and Wireless pH Monitoring
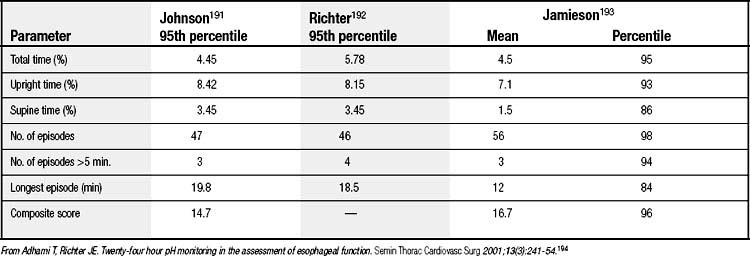
A pH below 4 has arbitrarily been chosen to define an acid reflux episode. The normal parameters for 24-hour pH monitoring, based on this reference, have been defined (Table 36-2). Total acid exposure time, expressed as a percentage of study time, is the best discriminator between normal and abnormal.15,16 Composite scores, such as the DeMeester score and the frequency-duration index, are no better than simple measured parameters in identifying abnormal reflux. The symptom index relates symptoms to reflux events and is calculated by dividing symptom episodes with reflux by the total number of symptom episodes multiplied by 100%; 50% is the optimum threshold.17
Wireless pH monitoring is a recent technological advance. The Bravo delivery system “pins” a 6 × 5.5 × 25-mm capsule to the esophagus 6 cm above the squamocolumnar junction, identified by endoscopy. Manometry is not required. Improved patient tolerance allows increased activities and improved food intake; moreover, monitoring is traditionally extended to 48 hours (Fig. 36-9).18 A 2008 American Gastroenterological Association Institute position paper states that wireless pH monitoring has superior sensitivity for detecting pathologic acid reflux.19 Drawbacks are cost, occasional premature detachment, and severe pain requiring endoscopic removal in less than 2% of cases. Like nasal pH monitoring, it is unable to detect nonacid reflux.
Impedance-pH Monitoring for Nonacid Reflux
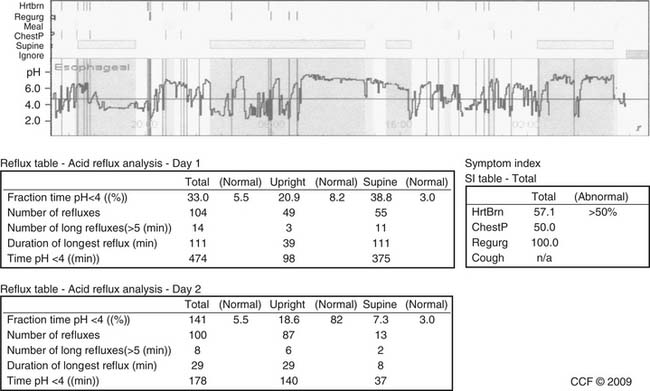
When an ionic fluid bolus traverses an electrode pair, impedance to current flow decreases. Impedance pairs placed on a pH catheter at multiple sites can detect retrograde fluid flow throughout the esophagus as acid reflux if the pH is less than 4, or nonacid reflux if pH is greater than 4.20 An expert panel concluded that impedance-pH monitoring when the patient is off proton pump inhibitor (PPI) therapy is the best test to detect all reflux episodes in a patient21 and thus is the best test for symptom association in patients with gastroesophageal reflux disease (GERD). However, it has limitations: a nasal catheter is necessary, and inaccuracies in current software require manual data correction of the study.
Impedance-pH monitoring when the patient is on PPI therapy has been used when suspected esophageal or extraesophageal GERD symptoms (or both) persist despite PPIs (Fig. 36-10). Small case series in patients with a positive symptom index have shown good results after fundoplication.22 However, we believe that long-term controlled studies, performed in centers with experience in impedance-pH monitoring, are needed to identify the role of such monitoring when only symptom association and nonacid reflux are found.
Endoscopic Esophageal Ultrasound
Endoscopic ultrasound (EUS) provides definition of the esophageal wall and periesophageal tissue not possible by routine fiberoptic esophagoscopy. It is indispensable in evaluating abnormalities of the esophageal wall and in diagnosing nonmucosal esophageal tumors. Ultrasound endoscopes scan the wall with ultrasound waves from 7.5- and 12-MHz. The probes, which can be passed through the biopsy channel of flexible endoscopes, can be used to evaluate esophageal strictures that prevent passage of standard ultrasound equipment. The esophagus and periesophageal tissues are seen as five alternating layers of different echogenicities (Fig. 36-11). This examination also images periesophageal structures, including regional lymph nodes. Both the layer of origin and the ultrasound characteristics of a mass are critical for diagnosing benign esophageal tumors. Periesophageal masses and regional lymph nodes can also be studied. EUS-directed fine-needle aspiration (FNA) provides cytologic and pathologic assessment of esophageal tumors, periesophageal masses, and regional lymph nodes.
Other Investigations
Bilitec 2000
The Bilitec 2000 fiberoptic spectrophotometer detects the presence of bilirubin, the principal component of bile, by absorption of the band of light (450 nm) characteristic for bilirubin.23 Because impedance-pH monitoring can detect nonacid reflux, the role of Bilitec in GERD is limited. Moreover, it is not widely available. However, measuring duodenogastroesophageal reflux may be useful in the patient with previous esophagogastric surgery and with suspected symptoms from bile reflux.
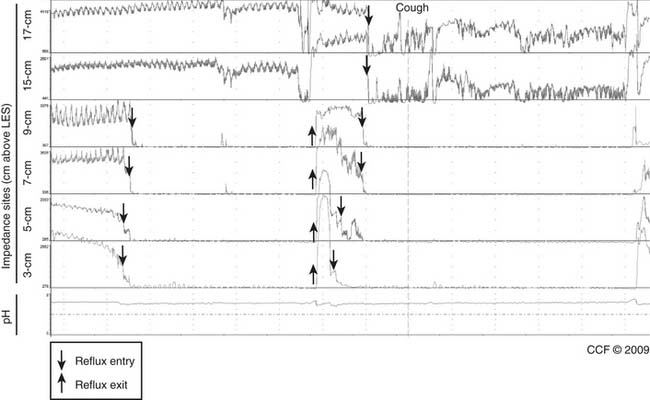
Impedance-Manometry
In impedance-manometry, multiple impedance electrode pairs on a manometry catheter allow transport of a swallowed bolus to be assessed as it traverses the esophagus (Fig. 36-12). Bolus transit can be compared with simultaneous esophageal peristalsis and contraction amplitude.24 Among 350 patients studied at a single center, all those who had achalasia and scleroderma had abnormal bolus transit, and 95% or more of those who had normal manometry, nutcracker esophagus, and LES dysfunction (high or low) had normal bolus transit. However, only 50% of patients with esophageal spasm and ineffective esophageal motility had normal bolus transit.25 Impedance-manometry may define different treatment strategies for patient subpopulations. For example, patients with abnormal rather than normal bolus transit may develop dysphagia after antireflux surgery, or patients with esophageal spasm and with poor as opposed to normal bolus transit may respond better to approaches improving esophageal emptying. Clinical studies with outcome data will assess if impedance-manometry improves patient management in these and other situations.
BENIGN ESOPHAGEAL DISEASES AND THEIR TREATMENT
Hiatal Hernia
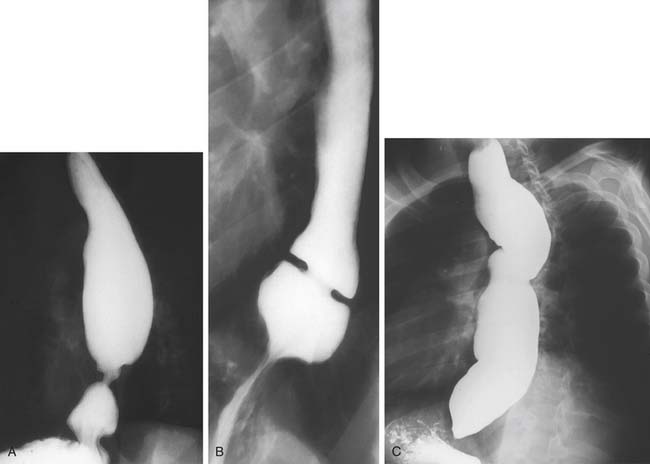
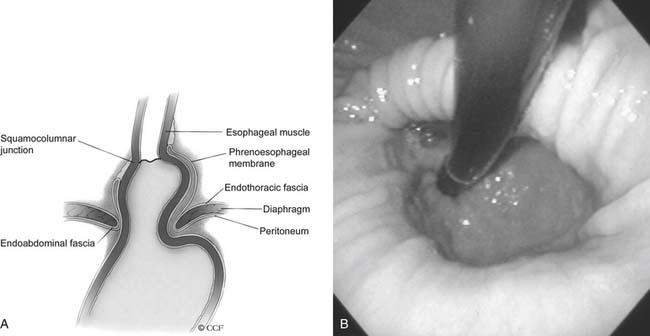
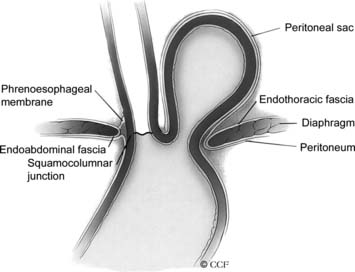
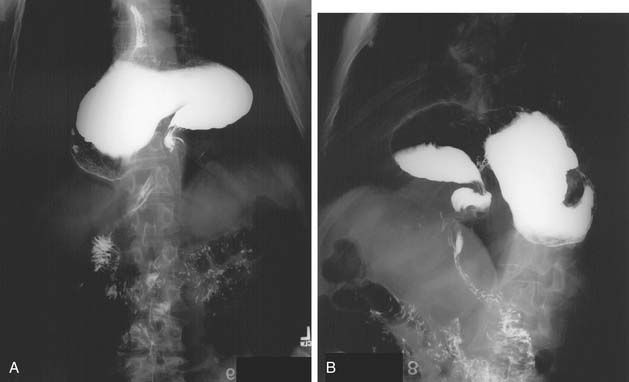
Herniation of abdominal contents through the esophageal hiatus is a common occurrence. With provocative maneuvers that increase intra-abdominal pressure, 55% of patients undergoing barium esophagram were found to have herniation of the stomach into the chest.26 Symptoms are secondary to reflux, incarceration or strangulation of herniated organs, or compression of thoracic structures. There are four types of hiatal hernia, each with its own symptom presentation. Type I, or sliding hiatal hernia, is the most common (Fig. 36-13, and see Fig. 36-6A). Herniation of the esophagogastric junction into the posterior mediastinum occurs because of thinning and elongation of the phrenoesophageal ligament. There is no potential for incarceration. The majority of patients with type I hiatal hernias are asymptomatic. If symptoms occur, they are related to GERD. Type II, or rolling, hiatal hernias are uncommon (Fig. 36-14). They result from a defect in or isolated weakness of the phrenoesophageal ligament, allowing a portion of the stomach to herniate through the hiatus while the esophagogastric junction remains anchored in the abdomen. Symptoms of gastric obstruction, strangulation, anemia, and, less commonly, shortness of breath and arrhythmia result from gastric herniation through the hiatus and presence of the stomach in the chest. Type III, or mixed, hiatal hernias are the second most common type (Fig. 36-15). Patients may present with reflux or with symptoms of type II hernias, or with both. As type III hernias increase in size, there may be organoaxial volvulus with the potential for strangulation. In many patients, these hernias may be a progression of type I hernias.27 Type IV hiatal hernias contain the stomach and other abdominal contents such as colon, spleen, small bowel, and pancreas (Fig. 36-16). The term paraesophageal hernia is sometimes used to describe any type II, III, or IV hiatal hernia.
Symptomatic hiatal hernias should be repaired. Repair of asymptomatic types II and III hernias is controversial. The potential for strangulation and gastric necrosis has been advocated as the prime reason to repair paraesophageal hernias in all patients, particularly because 50% mortality was initially reported when this complication occurred.28 However, strangulation is an uncommon occurrence without antecedent symptoms; therefore, this is an overestimate, and careful follow-up of asymptomatic patients is a viable alternative to repair in all patients.
Repair follows the principles of surgical management of GERD. Addition of a fundoplication is controversial, but it is definitely indicated if symptomatic GERD is present. Laparoscopic repairs have been reported to have an earlier and increased rate of failure.29 A meta-analysis showed this rate to be 25%, at variable follow-up periods.30 This high incidence of recurrence has prompted the use of a variety of mesh reinforcement of laparoscopic hiatal reconstruction. Although mesh has been reported to decrease recurrence at 6 months,31 the clinical significance of this finding32 and long-term durability33 are in question. In most patients, addition of a gastrostomy or gastropexy is not required. Compared with patients with GERD, patients with type III hiatal hernias are older and have more comorbidities. Adjusting for these factors demonstrates that patients with type III hernias are more likely to have pulmonary, thromboembolic, and bleeding complications in their postoperative course than those with type I hiatal hernias.34
Gastroesophageal Reflux Disease
GERD was defined at the recent Montreal consensus conference as “a condition which develops when the reflux of stomach contents causes troublesome symptoms and/or complications.”35 Typical symptoms of GERD are heartburn, regurgitation, and dysphagia. Extra-esophageal (or atypical) symptoms or disorders having an established association with GERD are cough, laryngitis, and asthma. Other atypical symptoms or disorders (pulmonary fibrosis, pharyngitis, otitis media, and sinusitis) are often linked to reflux and are proposed associations, but data are insufficient to establish causation.35 It is important that abdominal pain, gas, and bloating not be misinterpreted as GERD symptoms.
Complicated GERD includes reflux esophagitis, esophageal stricture, and Barrett’s esophagus. The mucosal response to acid may produce intestinal metaplasia (Barrett’s esophagus), and damage to the submucosa and muscularis propria can result in a short esophagus (see Figs. 36-4 and 36-6A).Two feared complications of GERD are peptic stricture and the development of high-grade dysplasia or intramucosal cancer in Barrett’s esophagus.
The LES and diaphragmatic hiatal mechanism are the major components of the reflux barrier.36,37 A hiatal hernia is seen in 50% to 90% of GERD patients.38–41 However, reflux events are most commonly caused by transient LES relaxations, although reflux over a low basal LES is also important.42 Poor acid clearance from peristaltic dysfunction and delayed gastric emptying contribute to prolonged acid exposure in some patients.
The mainstay of therapy for patients with GERD is medical management. PPIs can heal esophagitis in more than 90% of patients; randomized studies do not demonstrate a superiority of surgery over medical therapy.43–45 Atypical symptoms or disorders respond less predictably to PPIs, especially in the absence of typical symptoms. Controlled trials of PPIs showing benefit in patients with laryngitis or asthma have been predominantly in those having concomitant typical symptoms. However, a recent controlled trial in patients with chronic posterior laryngitis symptoms and endoscopic findings, but no typical GERD symptoms, found no benefit with esomeprazole.46
Surgical management of uncomplicated GERD should be considered in patients who are PPI intolerant and who have typical GERD symptoms responsive to PPI, and persistent typical GERD symptoms despite PPI, especially volume regurgitation.47 The following preoperative GERD evaluation is suggested. First, endoscopy should be performed to assess for presence of esophagitis, Barrett’s mucosa, stricture, hiatal hernia, and alternative upper gastrointestinal diagnoses such as ulcer disease. Second, manometry should be performed to locate and measure LES, to exclude another diagnosis such as esophageal spasm, and to assess peristalsis. Third, a barium esophagram should be performed to assess the anatomy of the esophagus and esophagogastric junction, mucosal changes, and esophageal function. Fourth, ambulatory pH monitoring off therapy should be performed to confirm excessive acid exposure. The presence of typical symptoms that respond to PPI therapy, and of abnormal acid exposure as determined by pH monitoring, are reliable predictors of successful surgical treatment of GERD.48,49 In patients with suspected gastric drainage abnormalities, a nuclear medicine gastric emptying study is required.
Surgical management is less likely to be effective in patients with persistent atypical symptoms or disorders in the absence of typical GERD symptoms. Thus, antireflux surgery primarily for atypical symptoms or disorders should be reserved for patients with concomitant typical GERD symptoms, and in whom causality of reflux for the atypical symptom or disorder has been established to the greatest degree possible during the preoperative evaluation just described. Although small case series have shown good results for antireflux surgery in patients with persistent cough on PPIs and reflux by impedance-pH monitoring,50 long-term controlled studies are needed to validate this approach.
Complicated GERD is first managed with aggressive medical therapy; however, peptic esophageal stricture and chronic Barrett’s ulcer may require surgery. The columnar cell–lined esophagus is not in itself an indication for surgery. It is debatable that surgery can reverse the changes of columnar lining. Partial reversal, although interesting, is not a compelling argument for surgical correction of GERD.51 Effective reflux control may reduce the incidence of malignant degeneration in the columnar cell–lined esophagus. However, failure of antireflux repairs is frequently seen in patients with adenocarcinoma of the esophagus. Patients with a columnar cell–lined esophagus have the most disordered physiology, largest hernias, and most disrupted hiatal mechanisms.52 Therefore, either prevention of or halting malignant degeneration in a columnar-lined esophagus is not an indication for surgery. If a patient with a columnar cell–lined esophagus has antireflux surgery, the need for endoscopic surveillance is not eliminated. Thus, the patient with a columnar cell–lined esophagus has the same indications for surgery as the patient with a squamous cell–lined esophagus.
The principles of antireflux surgery are restoration of the intra-abdominal length of esophagus, reconstruction of the esophageal hiatus, and reinforcement of the LES.53 These can be accomplished by a number of approaches and techniques. Possible approaches, in descending order of morbidity, are thoracoabdominal, thoracotomy, laparotomy, and laparoscopy. facility with all of these approaches is necessary.
Restoration of the intra-abdominal esophagus requires reducing the hiatal hernia and mobilizing the esophagus. This portion of the operation necessitates recognition of the short esophagus.54 Failure to lengthen the short esophagus by extensive esophageal mobilization to the aortic arch or by addition of a Collis gastroplasty will result in a repair under tension and early failure. The short esophagus should be suspected by history of a stricture or previous dilation or findings of a long segment of columnar cell–lined esophagus, a large type I hiatal hernia (>4 cm), a type III hiatal hernia, or failure of the hernia to reduce below the diaphragm on upright barium esophagram.
The importance of reconstruction of the hiatus cannot be underestimated. It plays a role in reflux prevention equal to that of the LES.55 The recent addition of mesh reinforcement of the hiatal closure ignores the past history of antireflux surgery and the dynamic nature of this structure. Complete mobilization of the hiatal crura and careful removal of the hernia sac will allow primary suture reconstruction of the hiatus. Failure to reconstruct the hiatus is a common cause of failure of laparoscopic antireflux surgery.56 The principal reason for reherniation after an otherwise adequate repair is tension on the repair, either because the short esophagus was ignored or because it was not recognized, and not an unbuttressed reconstruction of the hiatus.
The final step in the surgical correction of GERD is reinforcing the LES by constructing a fundoplication, which may be either total or partial. The Nissen fundoplication, a 360-degree total fundoplication, completely encircles the esophagus. Partial fundoplication is typically 270 degrees and is either anteriorly placed (Belsey Mark IV repair), or posteriorly placed (Toupet repair). Theoretically, the tradeoff is between better reflux control with a total fundoplication and less dysphagia with a partial fundoplication. Some surgeons tailor the fundoplication to suit the peristaltic activity of the esophageal body.57 High-resolution manometry should allow a more precise cutoff at which the severity of peristaltic dysfunction requires partial fundoplication. Until then, we consider total fundoplication to be appropriate for all except the patient with an aperistaltic esophagus.15,58–61 The use of a partial fundoplication for complicated GERD is associated with an increased incidence of failed repair.62 Dysphagia after surgery for GERD is usually transient with a properly constructed fundoplication. Prolonged dysphagia is indicative of a malformed fundoplication, either long, tight, or twisted. Return of normal esophageal motility with resolution of GERD after fundoplication is hypothetically possible. It is more likely that the amplitude of the peristaltic wave will increase than that failure of propagation will be corrected. Therefore, any fundoplication should be constructed under the assumption that the peristaltic abnormalities will not improve. Finally, the potential for postprandial symptoms of gas bloat and early satiety must be mentioned to any patient being considered for fundoplication.
An often ignored but essential part of the physical examination is measuring and recording weight and height and calculating body mass index (BMI). Overweight (BMI, 25 to 29) and obese (BMI, 30 to 34) GERD patients should be counseled on weight loss and encouraged to reach their ideal weight before elective surgery. Because obesity and GERD are interrelated,63,64 successful and sustained weight loss may eliminate the need for surgery. Although there is disagreement about the impact of obesity on the outcome of antireflux surgery,65–70 the health benefits of weight loss in severely (BMI, 35 to 39) and morbidly (BMI, ≥40) obese patients with GERD should make weight loss surgery the operation of choice in these patients.
Antireflux operations are not infinitely durable. It is important that all patients are instructed to avoid activities that excessively increase intra-abdominal pressure and to maintain their ideal weight.71 Unrealistic patient expectations and widespread application of laparoscopic fundoplication without careful patient selection in low-volume centers by inexperienced surgical teams have produced poor results and have had a negative impact on this operative approach.72–74
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree