Surgical Palliation of Inoperable Carcinoma of the Esophagus
Abbas Abbas
Carolyn E. Reed
According to the National Cancer Institute, the estimated number of new cases of esophageal cancer in the United States in 2007 was 15,560. At the same time, the expected number of deaths from the disease was 13,940. In the western hemisphere, the incidence of esophageal adenocarcinoma continues to rise annually. Even after definitive multimodality therapy with curative intent, the failure rate remains very high and most patients develop recurrent disease, with an overall 5-year survival rate of 14.9%. The usually late presentation and aggressive nature of this disease results in more than 50% of patients presenting with inoperable disease at the time of diagnosis, owing to metastases, locally advanced and unresectable tumor, or poor medical condition. In this group of patients, the average life expectancy is only 5 to 6 months, and the focus of care should shift to improving these patients’ quality of life and relieving their devastating symptoms.
Most of the symptoms of advanced esophageal cancer are due to either obstruction or fistulization. Dysphagia is the most common presenting symptom of advanced esophageal cancer, seen in almost all cases. It usually progresses rapidly within months from occasional difficulty swallowing solid food to complete dysphagia to both solids and liquids. Eventually, aspiration of saliva and pneumonia develop, in addition to dehydration, starvation and loss of weight. The inability to eat or even swallow one’s own saliva is one of the most distressing symptoms in medicine. It is for this reason that despite the almost universally poor prognosis for these patients, all possible effort should be made to try to alleviate dysphagia.
Aspiration from tracheoesophageal fistulization may occur either secondary to direct tumor invasion into the airway or as a complication of prior radiation therapy or even stent placement. Patients develop acute pulmonary symptoms and pneumonia.
Locally advanced and metastatic esophageal cancer is generally an inoperable problem. The high morbidity and mortality associated with surgical resection or radiation and chemotherapy for patients with advanced-stage cancer may cause the risks to outweigh the potential palliative benefits. Endoscopic techniques have recently revolutionized the management of these patients, providing a good palliation and safety profile. Methods of endoscopic palliation include ablative techniques such as laser, argon beam plasma coagulation, photodynamic therapy, and brachytherapy. They also include endoscopic dilation and stent placement. Stents have evolved from rigid plastic stents to self-expanding metal, plastic, or hybrid stents.
Dilation
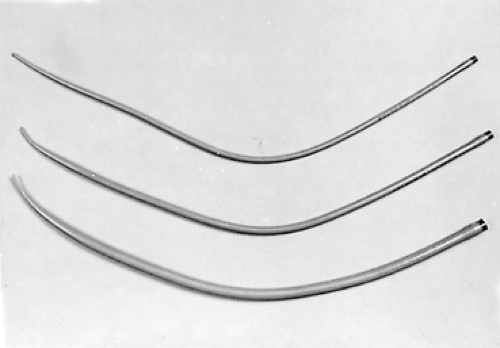
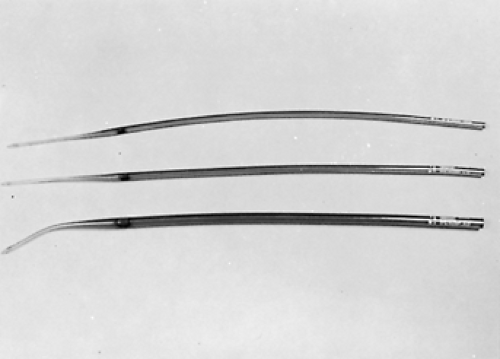
Simple repeated esophageal dilation is the oldest technique for palliation of dysphagia but is rarely associated with long-term relief. Usually, dysphagia recurs after only a few days or weeks.29 Dilation may be accomplished by using mercury-weighted rubber Maloney bougies (Fig. 160-1), wire-guided polyvinyl Savary dilators (Fig. 160-2), or hydrostatic dilating balloons (Fig. 160-3). Theoretically, the radial force of the dilating balloon is safer than the longitudinal force of bougies; however, this has not been compared in dilations of malignant strictures.27 Maloney dilators are passed blindly into the esophagus and are quite effective in experienced hands. We prefer using either wire-guided Savary bougies under fluoroscopic guidance or hydrostatic balloons under direct endoscopic visualization. The risk of perforation is higher in very tight lesions and in patients who have had previous radiation therapy. The procedure must usually be repeated every few days and is seldom sufficient in producing long-term relief. No large controlled trials have documented the
efficacy or complication rate of dilation of malignant strictures. With the availability of self-expanding metal and plastic stents, dilation is rarely used as the sole treatment for dysphagia.
efficacy or complication rate of dilation of malignant strictures. With the availability of self-expanding metal and plastic stents, dilation is rarely used as the sole treatment for dysphagia.
Fixed-Diameter Plastic Intubation Stents
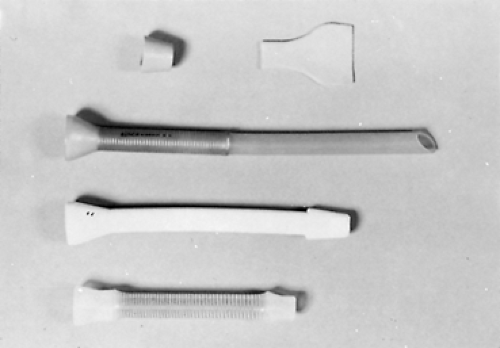
Prior to the advent of self-expanding metal stents (SEMS), the main methods for maintaining the lumen of the esophagus open was in the form of fixed-diameter plastic stents—e.g. the Celestin or Wilson–Cook stents (Fig. 160-4). These were inserted either using rigid esophagoscopy (push technique, Fig. 160-5) or by pulling through a gastrostomy (pull technique, Fig. 160-6). These tubes required dilation of the esophagus at least to their external diameter of 18 mm, which was associated with a high risk of perforation. Also their internal diameter was only 10 to 12 mm and therefore allowed only a liquid or very soft diet. The procedure-related complication rate is over 20% and includes perforation (8% to 12%), hemorrhage (2% to 5%), and fistula (1% to 3%). The mortality rate related to the procedure is 2% to 10%.37
Self-Expandable Metal Stents (SEMS)
SEMS were introduced in 1990, and have now replaced the conventional fixed-diameter plastic prostheses. Numerous studies have shown them to be associated with fewer complications and better efficacy (85%–100%) than fixed-diameter plastic stents.7,16,36 In addition they are much easier to insert, with a successful insertion rate of 95% and usually do not require a predilation unless the diameter of the lumen is narrower than that of the delivery system (6–11 mm). SEMS all have a thin wall and a large endoluminal diameter (16–23 mm), which ensures effective relief of dysphagia. These stents become embedded in the mucosa and are difficult to remove after several weeks.
There are several SEMS available which differ mainly in the type of alloy and the extent of covering (Table 160-1). The most commonly used SEMS are the covered and uncovered
Ultraflex II (Fig. 160-7, Boston Scientific) and the completely and partially covered Gianturco Z stent (Fig. 160-8, Cook Medical). The Wallstent II (Boston Scientific) is no longer produced. Several randomized studies have shown that these stents had equal efficacy in long-term relief of malignant dysphagia.35 A silicone or polyurethane coating allows stents to resist tumor ingrowth but makes them more prone to migration. Migration can be minimized by avoiding overdilation of the malignant stricture and placing the stent with at least 2 cm of overlapping length on both sides of the tumor.
Ultraflex II (Fig. 160-7, Boston Scientific) and the completely and partially covered Gianturco Z stent (Fig. 160-8, Cook Medical). The Wallstent II (Boston Scientific) is no longer produced. Several randomized studies have shown that these stents had equal efficacy in long-term relief of malignant dysphagia.35 A silicone or polyurethane coating allows stents to resist tumor ingrowth but makes them more prone to migration. Migration can be minimized by avoiding overdilation of the malignant stricture and placing the stent with at least 2 cm of overlapping length on both sides of the tumor.
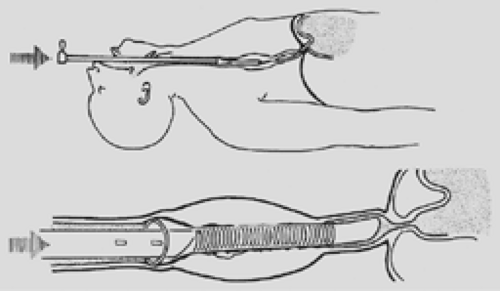 Figure 160-5. Push method with rigid endoscopic techniques. Note that the end of the tube is above the gastroesophageal junction to avoid reflux. This placement is preferred whenever possible. |
The stents all come preloaded on a small-diameter delivery system; when deployed, they exert outward radial force and expand to their full diameter. The radial force of the stent maintains lumen patency, and tumor compression holds the stent in place. They are all placed in a similar fashion, usually over a wire with endoscopic and/or fluoroscopic guidance. They are then deployed by a self-expanding mechanism. They may be placed under either general anesthesia or moderate sedation.
Early complications occur in approximately 10% of patients and include perforation, bleeding, pain, stent migration, and airway obstruction.33 Perforation is related to the use of excessive force during dilation or passage of the delivery system. Airway obstruction may occur, especially in the presence of large cervical or mediastinal adenopathy, which may impinge on the trachea after expansion of the stent. This may require emergent stent removal or even placement of a second airway stent.38
Late complications, including recurrence of dysphagia, may be seen owing to tumor overgrowth or ingrowth (10%–15%), granulation tissue hyperplasia, or stent migration (1%–15%).33,37 Other complications include food impaction, gastroesophageal reflux, esophagorespiratory fistula, and bleeding. Tracheoesophageal fistula can result from stent erosion through the esophagus and into the respiratory tree. Massive bleeding into the aorta may result from a similar process.
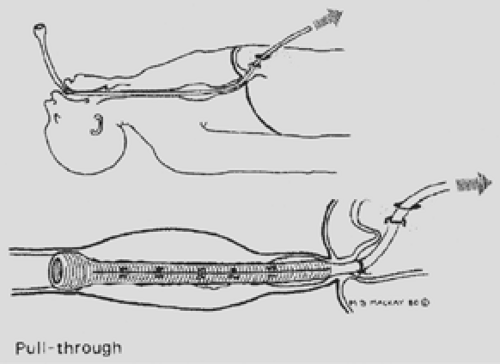 Figure 160-6. Pull method. Whenever possible, the tube eventually is cut off above the gastroesophageal junction. |
When tumor ingrowth or overgrowth does occur, it can be managed with several techniques, including placement of a second stent within the old one9,24 or using a variety of ablation methods including laser, argon beam plasma coagulation (APC), endoscopic debridement, and photodynamic therapy (PDT).
The ideal position for a stent is the midesophagus with both proximal and distal normal esophageal mucosa to allow incorporation of the flanges. However, for esophageal adenocarcinoma, it is usually necessary to place them at the gastroesophageal junction, where they may be more susceptible to migration because of the lack of distal attachment. At this location, stents also cause free gastroesophageal reflux; therefore, patients should follow antireflux precautions including proton pump inhibitors and head-of-bed elevation. Modified stents have been designed with a one-way valve to prevent this reflux. However, in different studies they have not been shown to be superior to unvalved stents in this regard. In the cervical esophagus, stents commonly cause
pain as well as a persistent foreign body sensation in the back of the throat. It is therefore not recommended to place a stent that is <1 cm from the cricopharyngeus. In such cases, a Montgomery plastic tube or the newer polyflex self-expandable plastic stent is preferable. These stents allow easy removal should the patient not tolerate them.
pain as well as a persistent foreign body sensation in the back of the throat. It is therefore not recommended to place a stent that is <1 cm from the cricopharyngeus. In such cases, a Montgomery plastic tube or the newer polyflex self-expandable plastic stent is preferable. These stents allow easy removal should the patient not tolerate them.
Table 160-1 Characteristics of FDA-Approved Covered Metal Stents | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||
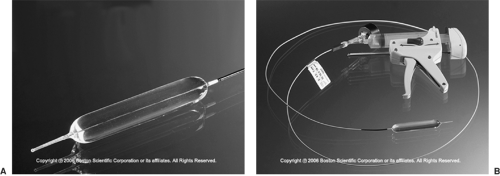
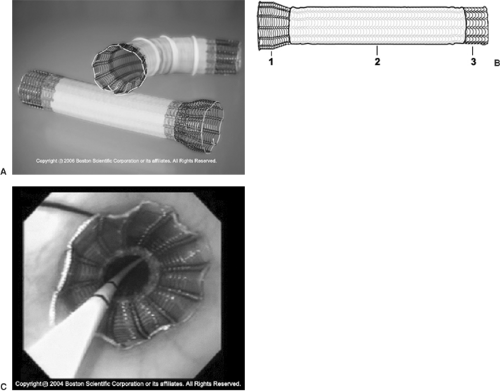 Figure 160-7. A: Self-expanding metal stent (Ultraflex). B: Diagram of stent construction. 1. large proximal flare. 2. polyurethane covering. 3. flexible knitted-loop design. C: Endoscopic view of deployed intraesophageal stent; notice the proximal uncovered flange. (With permission from Boston Scientific, Inc.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|