Chapter 21 Surgical Management of Aortoiliac Occlusive Disease
Arteriosclerotic occlusive disease of the abdominal aorta and iliac arteries is a common cause of ischemic symptoms in the lower extremities of middle-aged and elderly patients in the Western world. Although not as common as occlusive disease of the femoropopliteal arterial system, with which it may be combined, aortoiliac occlusive disease may be more disabling because of the greater number of muscle groups subjected to diminished perfusion. The initial manifestation of occlusive disease of the distal aorta or iliac arteries is intermittent claudication with symptoms involving muscles of the thigh, hip, buttock, and calf. Because the calf muscles are usually the only muscle groups affected by intermittent claudication caused by superficial femoral artery occlusion, the involvement of more proximal muscles in the symptom complex may help to distinguish aortoiliac occlusive disease from femoropopliteal occlusive disease. Unfortunately, a sizable minority of patients with aortoiliac disease complain only of calf claudication. In addition to claudication, male patients with aortoiliac occlusive disease may complain of difficulty in achieving and maintaining an erection because of inadequate perfusion of the internal pudendal arteries. The Leriche syndrome in males consists of the manifestations of aortoiliac occlusive disease and includes claudication of the muscles of the thigh, hip, and buttock; atrophy of the leg muscles; impotence; and diminished femoral pulses.1
Aortoiliac occlusive disease per se is rarely the cause of ischemia at rest or ischemic tissue loss, except by embolization. The collateral circulation that develops around the occlusive process in the aorta and iliac arteries is usually rich and sufficient to supply the lower extremities with adequate quantities of arterial blood to ensure good resting tissue perfusion. However, arteriosclerotic plaques in the aorta and iliac arteries may cause the so-called blue toe syndrome (i.e., microembolization of arteriosclerotic debris to the terminal vessels in the foot).2–5 Such symptoms can occur in a patient who otherwise appears to have adequate distal arterial supply, including palpable pedal pulses in some instances. Under these circumstances, a search must be made by angiography for a proximal source of microembolization.
When aortoiliac occlusive disease is combined with femoropopliteal occlusive disease—a finding more common in elderly patients—resting ischemia may result.6 As in any arterial system, tandem lesions in the arteries supplying the extremities are more significant than single lesions.
The risk factors for aortoiliac occlusive disease are those for atherosclerosis in general and include cigarette smoking, hypertension, elevated serum cholesterol, and diabetes.7–18 In our experience, patients reporting symptoms of claudication caused by aortoiliac occlusive disease are on average nearly a decade younger than those complaining of claudication from superficial femoral artery occlusion. However, patients with ischemia at rest from the combination of aortoiliac and femoropopliteal occlusive disease are generally in the seventh decade of life and are not notably younger than those who develop ischemic rest pain from femoropopliteal disease.
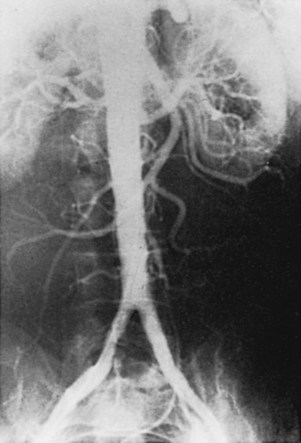
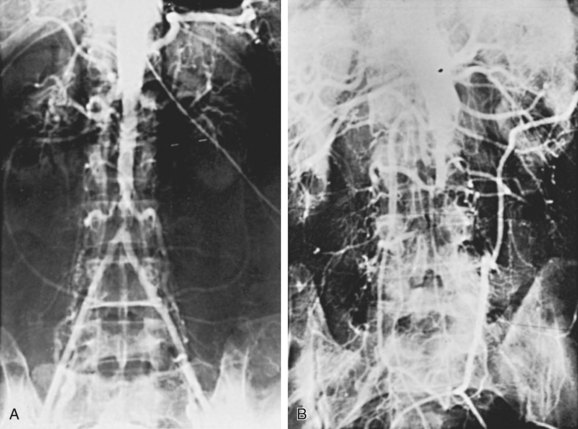
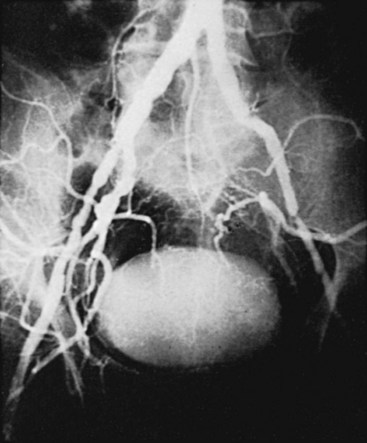
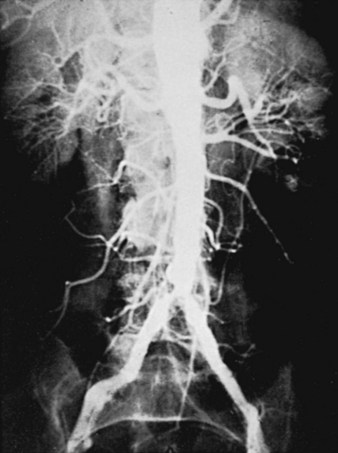
The initial lesions of aortoiliac occlusive disease usually begin at the terminal aorta and the proximal portions of the common iliac arteries or at the bifurcations of the common iliac arteries (Figure 21-1). The lesions then progress proximally and distally. Approximately 33% of the patients treated by authors for symptomatic aortoiliac disease have had disease at the origin of the deep femoral arteries in the groin, and more than 40% have had superficial femoral artery occlusions. The natural history of aortoiliac occlusive disease is one of slow progression.19,20 The ultimate anatomic result of aortoiliac atherosclerosis is variable, but can lead to occlusion of the distal abdominal aorta, with progression of the thrombus up to the level of the renal arteries (Figure 21-2). Although occlusion of the terminal aorta, once it occurs, may remain stable for years, it does not always have a benign course, as reported by Starrett and Stoney.21 They observed that more than one third of patients with aortic occlusion went on to show thrombosis of the renal arteries over a period of 5 to 10 years (Figure 21-3). However, Reilly and colleagues22 later suggested that the renal arteries remain patent; no instances of thrombosis occurred in 21 patients followed up with arteriography after a mean of 27.7 months.
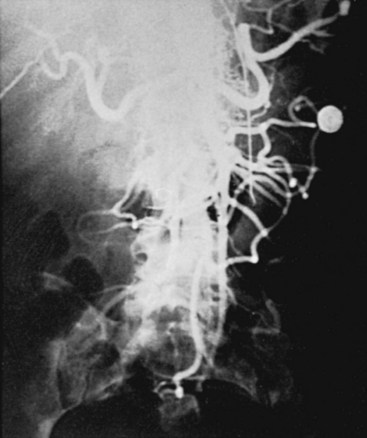
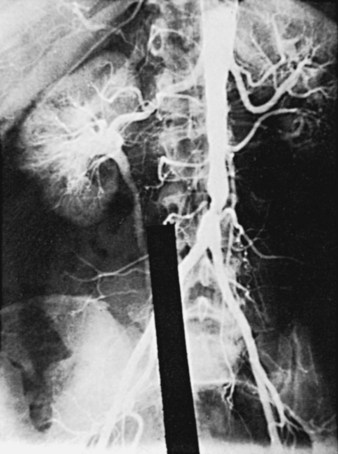
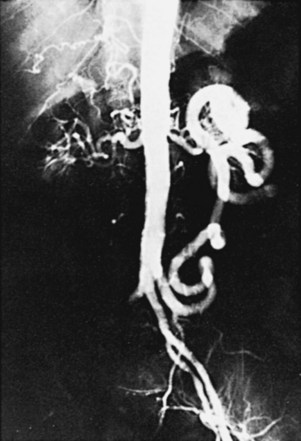
Variants in the pattern of aortoiliac occlusive disease occur, including relatively circumscribed occlusive lesions of the midabdominal aorta described in early middle-aged women who are heavy cigarette smokers (Figure 21-4). Although the upper abdominal aorta is ordinarily spared in patients with aortoiliac occlusive disease, a minority of these patients have marked involvement of this aortic segment, with occlusive disease at the origins of the major visceral vessels and renal arteries (Figure 21-5).
Diagnosis
The diagnosis of aortoiliac occlusive disease is ordinarily and easily made on the basis of the patient’s symptoms. Complaints of high claudication, with or without accompanying sexual dysfunction in males, certainly suggest this disease process. Claudication symptoms, however, must be distinguished from symptoms of nerve root irritation caused by spinal stenosis or intervertebral disk herniation, which may be associated with activity and relieved by sitting or lying down in some individuals.23 These patients can ordinarily be distinguished easily from patients with true claudication by the fact that their symptoms are produced as much by standing still as by walking and by the typical sciatic distribution of the pain.
Segmental Doppler pressures at all levels in the lower extremity are ordinarily lower than the brachial pressure. If no accompanying superficial femoral occlusive disease exists, no impressive gradient occurs between the high thigh pressure and the ankle pressure; however, disabling symptoms can occur in patients with aortoiliac disease who have resting ankle pressures in the near-normal range and a normal ankle-brachial pressure index. Thus, in evaluating these patients, repeating the pressure measurements after a period of graded exercise is often wise.24 A marked fall in ankle pressure immediately after exercise occurs if the patient’s symptoms are caused by significant aortoiliac occlusive disease. More sophisticated Doppler waveform analysis or the use of a pulse-volume recorder may reveal patterns suggestive of proximal occlusive lesions.25–27 We have found, however, that resting and postexercise Doppler pressure measurements are satisfactory for the evaluation of the majority of patients.
Preoperative Evaluation
Angiography has historically played a major role in the preoperative evaluation of patients with symptomatic aortoiliac disease and can generally be performed by the retrograde Seldinger technique using the femoral approach.28 When angiography is not possible, magnetic resonance angiography (MRA) is particularly well suited for evaluating the aorta and renal and iliac arteries. New and faster acquisition techniques and high-quality, three-dimensional postprocessing capabilities make MRA an attractive, noninvasive, and accurate alternative. In some institutions, computed tomography angiography is favored over MRA as a more clinically useful, noninvasive alternative to standard angiography. Regardless of the modality, the goal of the radiographic examination is to provide views of the entire abdominal aorta in two planes to demonstrate unexpected lesions of the celiac axis or superior mesenteric artery origins, to provide anteroposterior and oblique views of the pelvis to define any iliac artery lesions in more than one plane, and to demonstrate possible lesions at the origins of the deep femoral arteries. Views should also be obtained of the vessels in the thighs, at the knees, and in the calves to demonstrate associated femoropopliteal occlusive disease and the quality of the runoff. At the time of angiography, obtaining pull-back pressures across iliac artery lesions of doubtful significance is a useful technique because it can demonstrate whether such lesions are likely to interfere with flow. Measurements should be taken at rest and after papaverine injection or during a period of reactive hyperemia after tourniquet ischemia to mimic the hemodynamic situation that occurs during exercise.29 Intraarterial digital subtraction angiography has become quite useful for evaluation of the aortoiliac arterial segment. The advantages of this technique include the use of small amounts of contrast medium and good resolution of the vessels studied.
Percutaneous balloon therapy with or without stenting has supplanted surgical reconstruction as the most common therapy for aortoiliac occlusive disease. The recent TransAtlantic Intersociety Consensus (TASC II) document delineated which patients are best served by percutanous versus surgical therapy.32 It is generally believed that TASC types A and B lesions (focal, short-segment lesions [≤3 to 10 cm], unilateral or bilateral) are best treated with endovascular techniques. Conversely, TASC type D lesions (long-segment occlusions and diffuse, severe long-segment disease, particularly bilateral) are best treated with open surgery. Intermediate TASC type C lesions can be treated appropriately with either technique, but are increasingly being treated initially with percutaneous approaches.
Aortofemoral Bypass Graft
Over the past 2 decades, the aortofemoral bypass graft has remained the gold standard for the treatment of severe symptomatic aortoiliac occlusive disease. This procedure’s 30-day operative mortality rate of 5% to 8% in the early 1970s has been reduced in our own experience to less than 2% over the past 15 years, a level consistent with reports from other surgeons and similar to that observed in patients undergoing elective abdominal aortic aneurysm repair.33–39 Arterial insufficiency of the lower extremities is a manifestation of a systemic process that results in clinically evident coronary artery disease in approximately 50% of these patients.40–42 Reduced operative mortality has been observed and is associated with a concomitant reduction in the number of early cardiac deaths. The improved perioperative management of patients with diseased hearts has resulted from a number of factors, including selective employment of preliminary cardiac surgery for certain individuals, sophisticated pharmacologic management of the damaged myocardium, and more precise perioperative fluid management tailored to the individual patient’s myocardial reserve.39
Surgical Technique
The knitted Dacron prosthesis is the standard graft material used by most surgeons with experience in aortoiliac reconstruction. This material, usually impregnated with collagen or gelatin, may provide a more stable pseudointima than woven prostheses do.43,44 An important factor contributing to improved results has undoubtedly been recognition of the critical role of the deep femoral artery in providing sustained patency of the aortofemoral graft limb.33,36,45,46 The current practice of extending the distal anastomosis down over the origin of the deep femoral artery to ensure an adequate outflow tract has been widely accepted and is important in patients with tandem superficial femoral occlusions and in patients with stenosis of the deep femoral origin. We have found, however, that if extensive profundaplasty or endarterectomy is necessary, this vessel is better closed with an autogenous tissue patch of saphenous vein, bovine pericardial patch, or endarterectomized superficial femoral artery than attempting to make a long deep femoral patch with the distal end of an aortofemoral prosthesis.45
The incidence of graft infection has been minimized with preoperative and intraoperative antibiotics.33,47–49 Aortoenteric fistulas can be prevented by closure of retroperitoneal tissue and the posterior parietal peritoneum over the graft and proximal suture line to prevent erosion of the graft into the duodenum.33,50,51 The abandonment of silk sutures in favor of permanent prosthetic suture material has undoubtedly helped to reduce the incidence of false aneurysm formation.
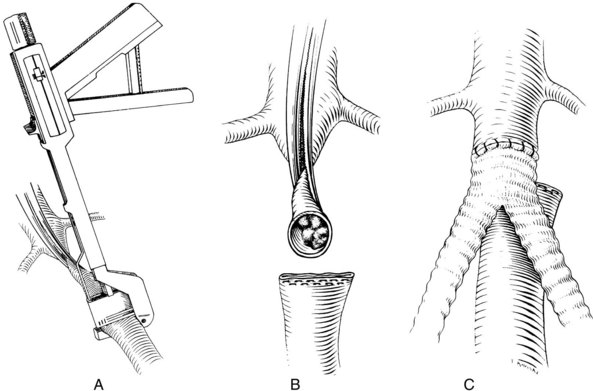
A good deal of controversy remains over the proper method of performing the proximal anastomosis of an aortobifemoral graft.33,52,53 Most surgeons favor the end-to-end technique of proximal anastomosis, with transection of the aorta between clamps approximately 1 to 2 inches below the renal arteries and oversewing or stapling of the distal end (Figure 21-6). This permits an endarterectomy or thrombectomy of the proximal aortic stump under direct vision before constructing the anastomosis. It also has the advantage of not requiring flow to be reestablished in the more distal aorta, where arteriosclerotic plaque and mural thrombus may have been loosened by application of the distal clamp. This may avoid intraoperative emboli to the lower extremities.
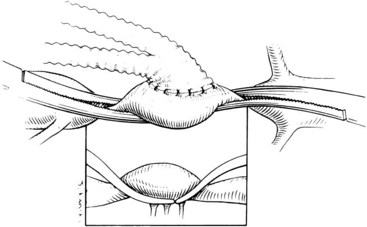
Some authors claim that the end-to-end technique reduces the incidence of aortoduodenal fistulas because the end-to-end anastomosis does not project anteriorly, as does an end-to-side aortofemoral reconstruction. Unfortunately, the controlled studies that are available do not substantiate that the results of the end-to-end technique are significantly superior to those of the end-to-side technique. Therefore the end-to-end technique is probably more appropriate for patients who will not suffer any hemodynamic disadvantage from interruption of forward flow in the abdominal aorta. This technique also appears to be desirable for patients who have already suffered complete aortic occlusion. The end-to-side technique (Figure 21-7) is reserved for individuals who would lose perfusion of an important hypogastric or inferior mesenteric artery if forward flow in the aorta were sacrificed at the time of surgery.52 Arteriographic studies in patients with indications for an end-to-side anastomosis are shown in Figures 21-5 and 21-8.
Although aortofemoral reconstruction has the potential to restore potency to males with sexual dysfunction because of inadequate hypogastric artery perfusion,52,53 surgical dissection in the area of the terminal aorta and proximal left common iliac artery can also cause difficulty with both erection and ejaculation by interfering with the autonomic nervous plexus, which sweeps over these vessels.54 When performing aortofemoral bypass grafting, confine the dissection of the aorta to the area between the renal arteries and the inferior mesenteric artery. The aorta is exposed anteriorly and laterally, without distorting the vessel, to avoid embolization of arteriosclerotic debris. After systemic heparinization, the distal clamp is placed proximal to the inferior mesenteric artery; then the aorta is cross-clamped below the renal arteries, where the aortic wall is likely to be considerably less diseased. The aorta is divided transversely, and the distal end is beveled and oversewn.
Despite significant advances in laparoscopic general surgery, applications of this new technique to vascular surgery have been few. However, several authors have applied laparoscopic techniques to aortofemoral reconstruction. Whether performed completely via the laparoscopic approach or through limited incisions with laparoscopy-assisted dissection, the procedure has proved to be time-consuming and technically challenging.55,56 As the technology evolves and intracorporeal anastomotic techniques are refined, however, the role of laparoscopic aortofemoral bypass will expand and become clearer.
In patients with well-localized aortoiliac lesions, aortoiliac endarterectomy may be a suitable option. This operation, even in the hands of enthusiasts, is confined to patients whose disease ends distally near the bifurcation of the common iliac arteries (Figure 21-9; see also Figure 21-4).33 Endarterectomy of the external iliac artery is tedious and unrewarding for most surgeons. There is little evidence to suggest that endarterectomy is superior to a properly performed aortofemoral bypass graft in terms of early or late results. In our practice, aortoiliac endarterectomy is confined to a minority of individuals who appear to have principally aortic disease with little involvement of the iliac arteries. This group of patients characteristically consists of early middle-aged women with occlusive disease of the midabdominal aorta that ends at the aortic bifurcation or in the proximal portions of the common iliac arteries. We avoid aortoiliac endarterectomy in males because of concerns about interfering with the autonomic nerves at the terminal aorta and proximal left common iliac artery. Currently, most patients who would be considered candidates for aortoiliac endarterectomy from an anatomic standpoint are treated with percutaneous balloon angioplasty with or without stenting.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree