In a normal, healthy person, pleural fluid amounts to less than 1 ml. The early simple exudative stage (Stage I) occurs within the first 2 weeks of the developing empyema. The pleural fluid is increased in quantity; however, it has normal pH, with glucose level equivalent to serum level, low white cell count and absence of micro-organisms. If the patient is adequately treated with antibiotics, the exudate normally will resolve spontaneously at this stage, with preservation of the thoracic dimensions. However, if there is persistent infection, it may progress to the fibrinopurulent stage (stage II) between week 1 and week 6. This stage is characterized by neutrophil phagocytosis, with inhibition of plasminogen activator and decreased tissue-type plasminogen activator leading to increased fibrin deposition, low pH, low glucose (< 2.2 mmol/L) and high lactase dehydrogenase (> 1000 IU/L). This is typically manifested by thick and purulent secretions with formation of septae causing loculations. The final phase, which develops from week 5 onwards, the organizing phase (stage III), is characterized by formation of a fibrous peel which leads to restriction of lung expansion.
The bacteriology of empyema has changed with the advent of antibiotic therapy. In the pre-antibiotic era, Streptococcus was the most common organism. Currently in community-acquired pneumonia, the majority of the infections continue to be caused by gram-positive aerobes or facultative organisms. Streptococcus and Staphyloccus aureus account for 65% of isolated organisms. Streptococcal organisms include Streptococcus pneumoniae, beta-haemolytic streptococci group A and Streptococcus ‘milleri’ groups. Commonly isolated gram-negative bacilli include Escherichia coli, Klebsiella pneumonia, Haemophilus influenzae and Pseudomonas aeruginosa. Staphylococcus aureus accounts for 50% of hospital-acquired empyema. Anaerobic organisms account for 13% of isolates. The frequency of pathogens such as anaerobic cocci, Fusobacterium and Bacteroides species is increasing in the community-acquired infection. These organisms are particularly seen in association with aspiration pneumonia and lung, dental and oropharyngeal abscess. The remainder of cases are due to mixed aerobic and anaerobic isolates (23%)[6]. In many cases no organism is ever isolated, which may relate to early speculative antibiotic use.
Diagnosis
Empyema should be suspected in patients presenting with acute respiratory illness associated with pleural effusion. Symptoms and signs include dyspnoea, cough, pleuritic chest pain, fever, tachycardia, malaise, anorexia and weight loss. Extension of a pleural infection, outside of the thoracic cage and into the neighbouring chest wall and surrounding soft tissues, is known as empyema necessitans. Empyema necessitans may be observed in patients in whom presentation is late or in association with particular organisms such as Mycobacterium tuberculosis and Actinomyces species. Mycobacterium tuberculosis account for approximately 70% of cases[7] and Actinomycosis for most of the remainder.
Investigations should include haematology, biochemistry and cultures of blood and sputum for aerobic and anaerobic bacteria. There are seven clinical variables that are found to be positively predictive of pleural infection: albumin < 30 g/L, CRP > 100 mg/L, platelet count > 400 × 109/L, sodium < 130 mmol/L, intravenous drug and alcohol abuse.
New biomarkers such as tumour necrosis factor alpha (TNF-α), myeloperoxidase, matrix metalloproteinase-2, interleukin 8 and CRP may have potential as future biomarkers in the diagnosis and management of empyema[3].
Imaging
Radiological investigation in empyema facilitates image-guided aspiration for diagnostic purposes, is an aid to chest drain insertion and allows assessment of loculations to guide relative merits of drain insertion versus operative intervention. Chest radiograph is the first-line imaging investigation. A lateral chest radiograph may help distinguish between free-flowing or loculated collection in the pleural space. CT, however, may be more useful in aiding the diagnosis of empyema, as well as staging the disease, as it facilitates differentiation between pleural effusion, lung abscess and consolidation. Classically, empyemas are usually posterolateral, and most extend to the diaphragm. CT also allows better visualization of septations, thickness of pleura, the presence or absence of trapped lung and associated underlying abnormalities.
Ultrasound (US) is able to differentiate between pleural effusion and consolidation, facilitates image-guided aspiration of effusion and is superior compared with CT in the assessment of loculations. US-guided aspiration will yield higher pleural aspirates and a lower risk of organ perforation compared to non-image-guided aspiration. Although US and CT have established roles in the investigation of parapneumonic effusions, neither technique reliably identifies the stage of pleural infection or predicts those patients who subsequently require surgical intervention after failed management by chest tube drainage[8].
Pleural aspiration
US-guided pleural aspiration is recommended in effusion > 10 mm in depth, especially if associated with pneumonic illness, ongoing sepsis or recent history of chest trauma or surgery. Frank pus indicates the need for formal drainage. Anaerobic pus is usually foul-smelling, whilst aerobic pus usually has little or no odour. Biochemical analysis (pH, glucose and protein concentration), microbiological culture and cytology are indicated. Pleural pH of < 7.2 is the single most powerful predictor of the need for chest tube drainage. Glucose level of fluid < 400 mg/L, LDH above 1000 IU/ml, protein level > 3 g/ml and WCC > 15,000 cells/mm[3] are also consistent with infection of the pleural space. Microbiological yield for suspected empyema is usually low. In the MIST-1 (Multicenter Intrapleural Sepsis Trial), positive pleural culture was only obtained in about 15% of patients. Polymerase chain reaction and immunological analysis may also identify the offending organisms.
Underlying causes of empyema should be sought. In patients with anaerobic empyema, examination of the oropharynx should be undertaken to exclude periodontal and oropharyngeal abscess. Bronchoscopy should be considered to exclude foreign bodies or endobronchial tumours. Bronchoscopy also facilitates bronchoalveolar lavage, which will increase the rate of isolation of positive microbiological culture when performed in addition to pleural fluid microcopy and culture.
Management
The principles of empyema management are drainage and sterilization of the pleural space, obliteration of empyema cavity, lung expansion and nutritional support. However, individual therapy should be tailored to the cause, clinical stage, underlying lung, presence or absence of bronchopleural fistula and patient’s clinical and nutritional status.
Drainage of pleural fluid
Presentation of patients in the earliest stage facilitates conservative management without empyema drainage. However, the presence of frankly purulent or turbid fluid, presence of bacteria confirmed with Gram stain, pleural pH < 7.2, poor clinical progress with signs of ongoing sepsis despite antibiotic therapy and loculated effusion are indications for closed tube drainage of empyema. The chest drain should be inserted under image guidance. There are no substantial data to recommend the optimal size of the chest drain or management of the chest drain such as regular flushing or suction. Historically, large-bore surgical chest drains were used, but currently, there is an increasing use of small intercostal chest drains, which are inserted percutaneously. Small-bore catheters (10–14F) facilitate ease of insertion, and evidence for the optimal size of chest tube is scarce[1].
Antibiotics
British Thoracic Society guidelines recommend that all patients should be treated with adequate and appropriate antibiotics, guided when available by culture based on pleural and blood culture results, and microbiological review taking into account local patterns of resistance[1]. Antibiotic therapy should cover anaerobic infections, except in patients with culture-proven Streptococcus infection. Penicillins, penicillins combined with β-lactamase inhibitor, metronidazole and cephalosporins penetrate the pleural space well; aminoglycosides should be avoided due to poor pleural penetration. In the absence of positive culture results, antimicrobial therapy should be directed based on local hospital policy and resistance pattern. Antimicrobial therapy may be needed for 2 to 3 weeks; however, the ultimate duration should be guided by clinical progress. Intravenous treatment is recommended initially, with change to the oral route once the patient shows improvement.
Intrapleural fibrinolytics
In an attempt to improve drainage of empyema, fibrinolytics have been utilized intrapleurally to lyse fibrinous septations. A number of case series and small trials suggested that these agents may improve outcome. However, the MIST-1, a large multicenter UK trial, showed no overall benefit in using streptokinase, when given intrapleurally. This large study enrolled 454 patients randomized in doubleblind fashion to intrapleural streptokinase twice daily for 3 days or placebo. No benefits were noted in either mortality or the need for surgical intervention at either 3 or 12 months after randomization with intrapleural streptokinase compared to placebo (31% streptokinase group versus 27% placebo group). Moreover, patients receiving streptokinase had a greater tendency to suffer serious adverse effects such as chest pain, fever, rash and allergy (7 versus 3%, RR = 2.49, 95% CI 0.98–6.36, p = 0.08)[9]. Following this study, fibrinolytics alone fell out of favour for the management of adult empyema. More recently, the MIST-2 of four study treatments – intrapleurally tissue plasminogen activator (t-PA), DNase, each agent alone and double placebo – showed intrapleural t-PA–DNase therapy improved fluid drainage in patients with pleural infection with improvement in chest radiograph. Secondary outcome measures reduced frequency of surgical referral, and the duration of hospital stay was also improved. Treatment with either agent alone was ineffective[10].
Prognosis
The prognosis is generally good in young and otherwise fit patients, especially if treatment was initiated early. There are rarely any long-term functional sequelae, although radiographically, there may be residual pleural thickening. Very rarely, a patient could develop fibrothorax with impairment of respiratory function.
Overall, however, the mortality and morbidity associated with the condition remains high, with 20% mortality if not effectively treated, as many of those affected are elderly with significant co-morbidities.
Indications for surgery
Surgery is indicated in patients with persistent sepsis and pleural effusion despite adequate therapy. Surgical options will be discussed in the next section. However, if surgery is not a feasible option due to the patient’s clinical condition, re-imaging with consideration for further chest drain insertion, with or without intrapleural fibrinolytics, may be considered.
Operative approaches (Figure 9.2)
Stage I: Drainage
Antibiotic therapy and simple drainage by either aspiration or intercostal chest drain are most useful in the early stage I exudative phase of empyema. If this is used as a treatment modality in primary thoracic empyema, the success rate ranges from 67 to 74%[11–13]. The thoracic surgeon rarely meets the patient at this early stage as patients are adequately managed by respiratory physicians.
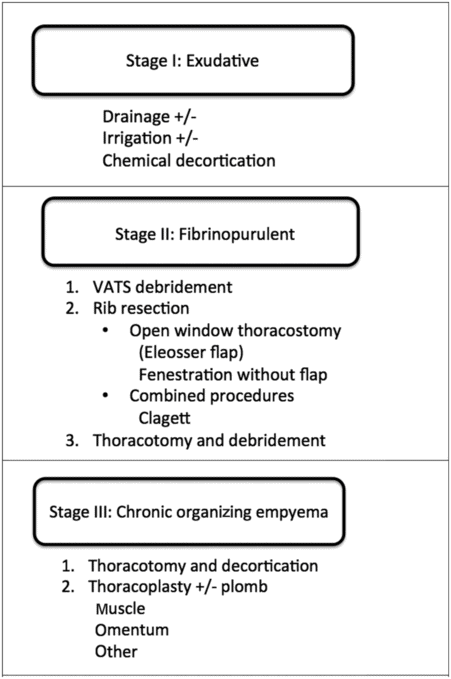
Summary of management of the three stages of empyema.
Stage II: Debridement
When the disease progresses to stage II, simple drainage alone is inadequate to resolve the infection, and debridement is then necessary to achieve local control. Surgical debridement of the pleural cavity can be carried out via open or video-assisted thoracoscopic surgery. Thoracoscopic debridement has gained popularity since the late 1990s with the general benefits associated with its minimally invasive nature compared to open, such as reduced post-operative pain and analgesic requirement, decreased air leak, decreased duration of chest tube drainage and shorter post-operative hospital stay.
In early stage empyema (stage II), two small randomized, controlled studies comparing VATS debridement with conservative drainage in patients with early/mixed-stage disease reported success rates of 83–93% in patients treated with VATS[14,15]. In other small case series, the reported conversion rates to open thoracotomy with VATS increased with increasing stage of the disease: 3–28% in early stage, 15–21% in mixed stage and 7–44% in late disease[16–26]. Between days 2 and 16 from presentation of empyema, the conversion rates to open with VATS increased from 22% to about 86%. A recent review of VATS decortication compared to open decortication in adults with primary empyema found that in stage II empyema, VATS is superior in the treatment of empyema with reduced post-operative morbidity, complications such as pain and air leak and reduced length of hospital stay[27].
The other major factor ensuring a higher success rate of treating empyema with VATS is the underlying condition of the parenchymal lung. In patients with empyema secondary to trauma, underlying normal lung will facilitate lung re-expansion with VATS drainage and debridement, hence preventing persistent space and infection in the pleural cavity.
Stage III: Decortication
Once the disease progresses to the advanced stage of organizing fibrous tissue encasing the underlying lung, decortication is usually a necessary therapeutic intervention. Decortication is defined as removal of constricting peel over the lung. It is usually indicated if the history of empyema is longer than 6 weeks, diagnostic assessment suggests progression to stage III disease and the patient is symptomatic. In these patients, lung perfusion can decrease by 20–25% in the affected lung, and decortication can improve vital capacity from 62 to 80% and forced expiratory volume in 1 second (FEV1) from 50 to 69%[28]. There is, however, controversy regarding the optimal timing of decortication. Advocates of early surgery will argue that there is less injury to the underlying lung parenchyma with early surgery as there is less significant ingrowth of fibrous tissue. However, on the other hand, late decortication after 3 months may achieve maximal functional respiratory recovery. The presence of intact visceral pleura, expandable lung and pleural space that will be completely obliterated by lung expansion are vital to the success of the therapy.
The mortality associated with decortication is high, ranging between 1.3 and 6.6%, with significant morbidity such as persistent air leak, bleeding, prolonged intubation with mechanical ventilation, persistent infection with prolonged ITU and hospital stay[11,29]
Many surgeons now advocate the use of VATS in decortication, even at the advanced stage. Many series and reviews suggests that VATS is as safe and effective in treating stage III empyema, with the added advantage of less post-operative pain, superior functional recovery of respiratory function, safer in high-risk patients and shorter hospital stay. Readers are directed to many good series and reviews of comparison between VATS and open decortication[16,17,19,30–32]. The conversion rate for patients treated with VATS decortication to open thoracotomy is between 3.5 and 41%. The main aim is to achieve sufficient decortication to allow full expansion of the underlying lung. VATS may be attempted in suitable patients; however, if this technique does not result in the desired outcome, open decortication will be required.
Management of persistent pleural space
Thoracoplasty
Thoracoplasty or collapse therapy was first introduced at the end of the nineteenth century, but due to its mutilating nature and being a poorly tolerated procedure, it rapidly lost popularity as a therapeutic option. However, with the advance in other aspects of surgery such as improvement in thoracic anaesthesia, blood transfusion and ITU support, thoracoplasty may be a good surgical option to treat a persistent pleural space in empyema in highly selected group of patients. The aim of thoracoplasty is to decrease the distance between the lung parenchyma and the chest wall. Collapsing the chest wall and filling the space with viable tissue such as omentum or viable muscle flap can achieve this. The modern indications, as described by Botianu, are
The lack of dissecting plane for decortication
Inability of the underlying to fully re-expand following decortication
Post-operative empyema, when decortication has failed or is not possible
Presence of bronchopleural fistula (BPF) – as this has to be dealt with by safe closure and suture reinforcement with a muscle flap
Presence of unresectable lesion in the lung parenchyma
Modern technique has achieved much better results due to an improved understanding and experience in raising muscle flaps and reducing the extent of chest wall mutilation by limiting the extent of rib resection. Meticulous assessment and planning pre- and peri-operatively are absolutely essential. With good planning and management, the operative mortality reported in the recent years is around 5%[33–37], with varying length of hospital stay and post-operative morbidity. Success rates of >90% have been reported when the procedure was performed in suitable patients.
Open window thoracostomy/fenestration
In debilitated patients who are unable to tolerate a major thoracic procedure as described earlier to manage a persistent pleural space, open window thoracostomy drainage is a useful option. The cavity is marsupialized via rib resection and open drainage. This long-term treatment plan involves multiple outpatient attendances for dressing changes and irrigation of the cavity. The cavity may eventually heal with time, fill with granulation tissue or require undergoing surgical closure.
Eloesser described a procedure involving a U-shaped incision through skin, subcutaneous tissue and muscle down to the ribs, creating a soft-tissue flap[38]. The exposed two or three adjacent ribs should be resected into 5-cm-long segments to fashion an approximately 5 to 7-cm opening to prevent contraction and spontaneous closure. Following drainage of empyema, the superiorly attached skin flap is folded inward underneath the chest wall and sutured both to the parietal pleura and empyema peel, and the cavity packed.
Theron Claggett described a similar two-stage open procedure in 1963. The superficial fascia is sutured down to the periosteum of resected ribs to leave a large window for daily irrigation and delayed closure.
Simple rib resection to facilitate adequate drainage of the empyema space is another alternative in high-risk patients who are medically unfit for major surgery. It is a minor procedure, performed under general anaesthesia. A short segment of the rib over the most dependent part of empyema is resected, with the space opened and deloculated, followed by insertion of either a large-bore chest drain or multifenestrated tube into the cavity to ensure adequate drainage. Mortality of rib resection in empyema reported in various small retrospective series ranged between 5 and 14%, with a low failure rate of <10% and median hospital stay of between 11 and 21 days[11,39,40]. However, in cases of late referral to a thoracic surgical unit for management of chronic empyema, Cham and associates reported a failure rate as high as 83% in patients who initially underwent rib resection subsequently requiring decortication[41].
Post-pneumonectomy empyema
Post-pneumonectomy empyema (PPE) is a serious complication of pneumonectomy with high morbidity and mortality in excess of 10%[42]. (See also Chapter 20 on BPF.) PPE must be treated immediately. However, there are various techniques to manage PPE; it very much is dependent on the presence of bronchopleural fistula and the patient’s general condition. Conservative treatment with chest tube drainage only seemed to result in a low success rate[43,44]. Surgical debridement, via either VATS or open thoracotomy, is associated with low mortality[45–48]. Even when more complex thoracoplasty was required to manage PPE, especially in the presence of BPF, the outcome reported was encouraging, with a success rate of between 81 and 100%[49–53]. A recent review by Zahid and colleagues found that open surgery is superior to minimally invasive management (which the authors have included chest tube drainage with or without chemical irrigation and video-assisted thoracoscopic debridement) in reducing empyema recurrence rate, mortality and re-intervention rate[54].
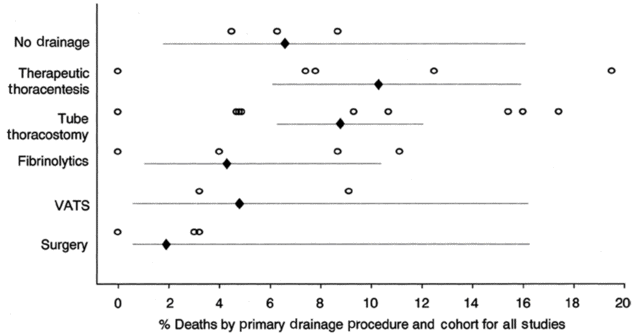
A study by Colice demonstrated the mortality associated with the natural history of the condition and inability to perform aggressive intervention[55] (Figure 9.3). Escalating mortality rate can be observed with reduction in intervention. Partly this reflects the fitness of the patients to undergo aggressive surgical intervention; it also serves to demonstrate the mortality of the condition when it is untreated or there is inability to undertake the treatment due to the patient’s condition.
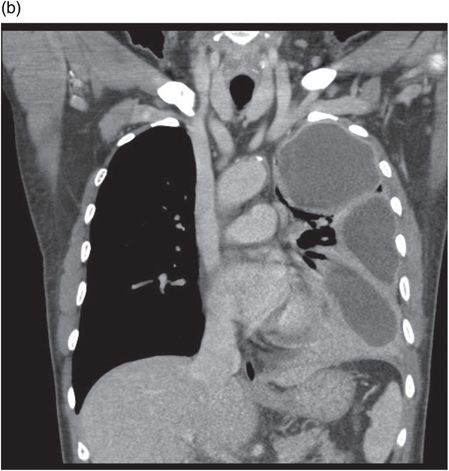
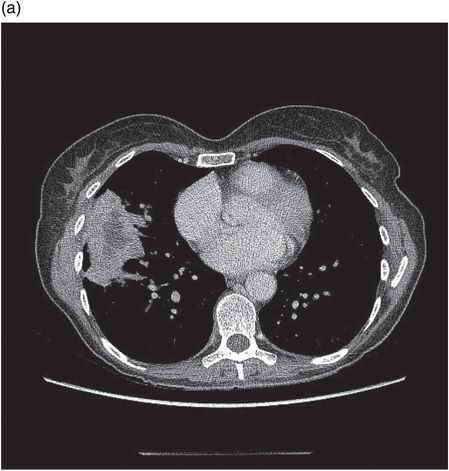
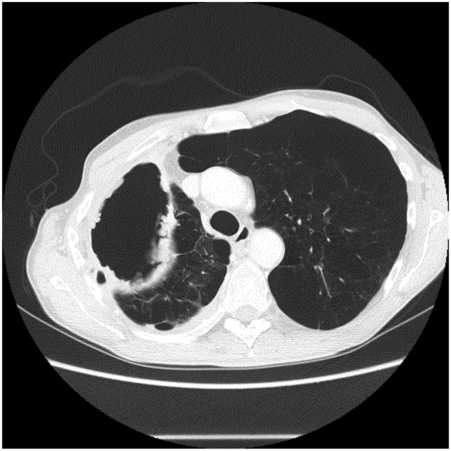
Chest CT demonstrating loculations prior to VATS drainage. No organism was isolated in this patient despite culture of pleural fluid.
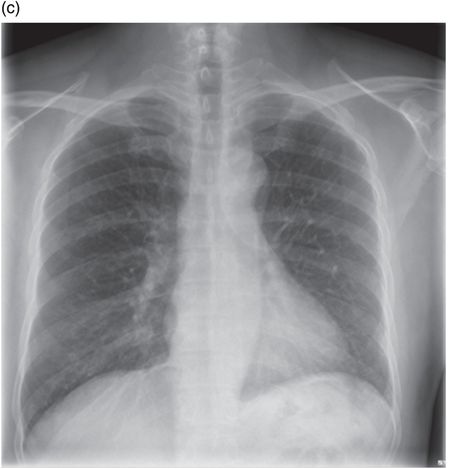
Post-operative chest radiograph following VATS and drainage of empyema.
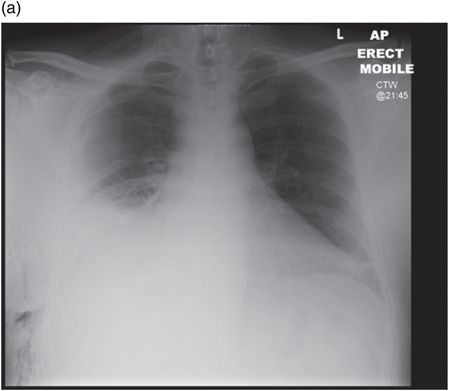
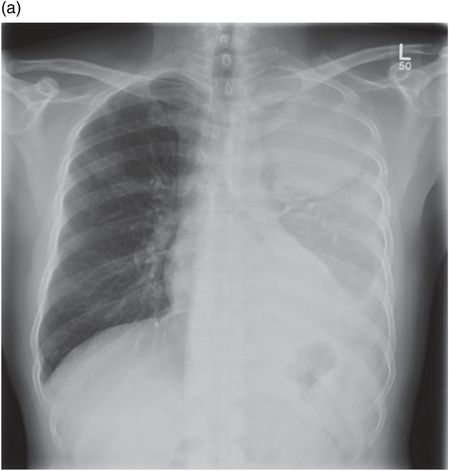
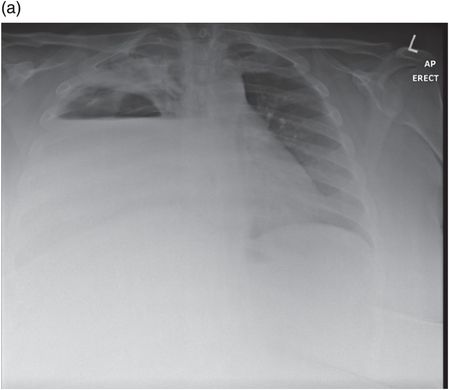
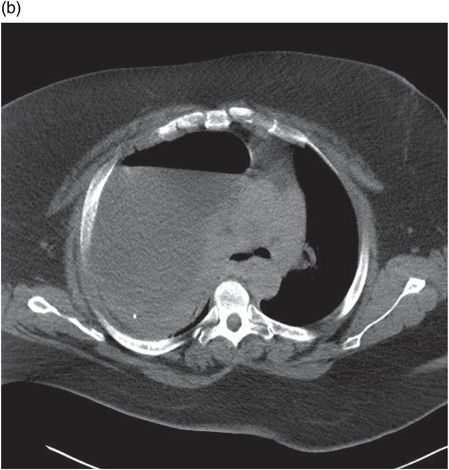
Chest radiograph demonstrating right lower zone opacification despite drain insertion. Patient presented with staphylococcal empyema following basal segmentectomy for metastastic thyroid carcinoma.
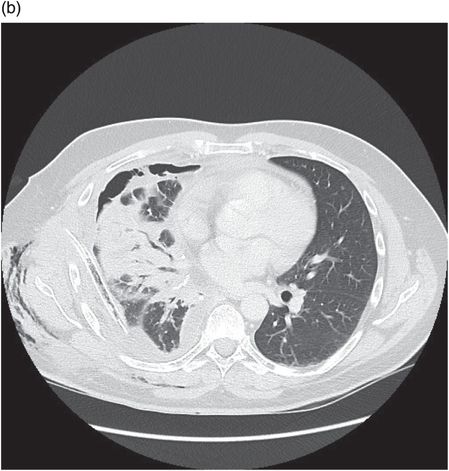
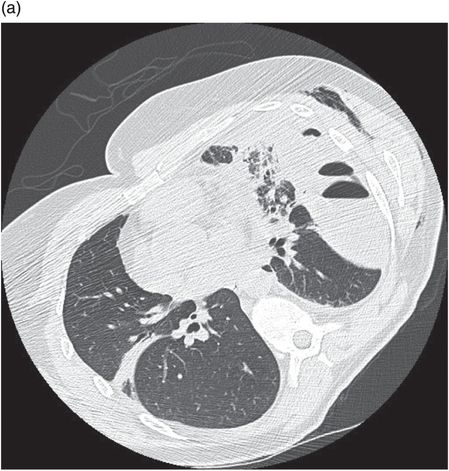
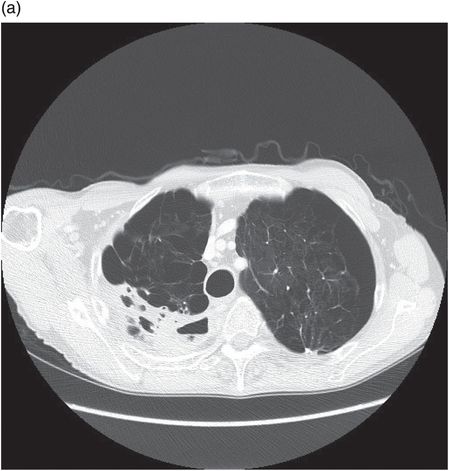
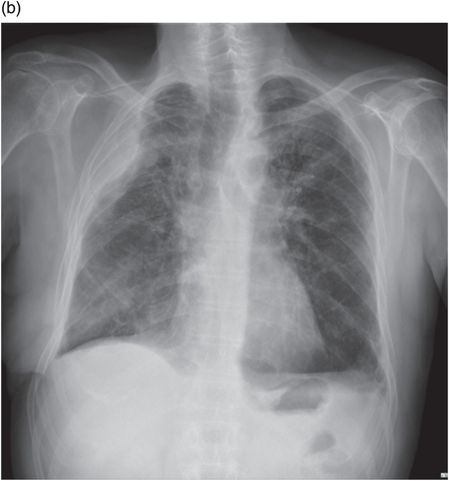
Chest CT showing loculated empyema with hydropneumothorax 6 weeks following aspiration of Streptococcus pneumoniae parapneumonic effusion.
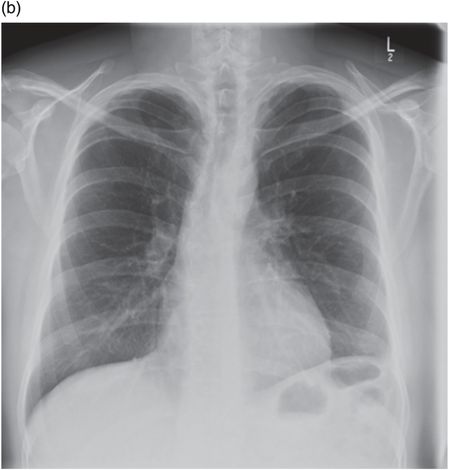
Chest radiograph of same patient 2 months following VATS and drainage of empyema.
Chest radiograph showing large right hydropneumothorax secondary to ruptured pulmonary abscess. Patient presented with sepsis and multiple organ failure requiring admission to intensive care.
Chest CT demonstrating large right hydropneumothorax with contralateral mediastinal shift. Patient underwent drainage of 2.5 L of pus from pleural cavity and cavernostomy of underlying lung abscess.
Chest CT of patient presenting with complications of right lung abscess. Abscess was associated with bronchopleural fistula, empyema and contamination of bilateral lung parenchyma. Patient developed acute respiratory distress syndrome requiring prolonged intubation and ventilation.
Follow-up chest radiograph showing resolution of changes and opacification of right upper zone in area of interposition muscle flap.
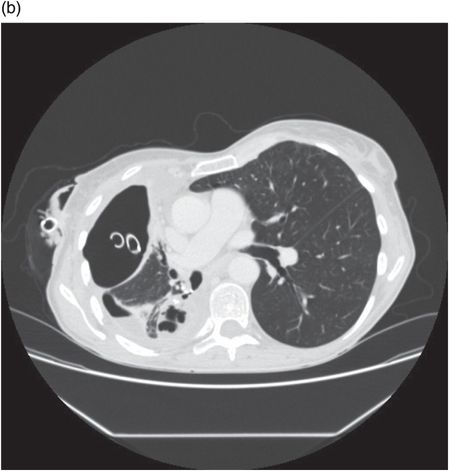
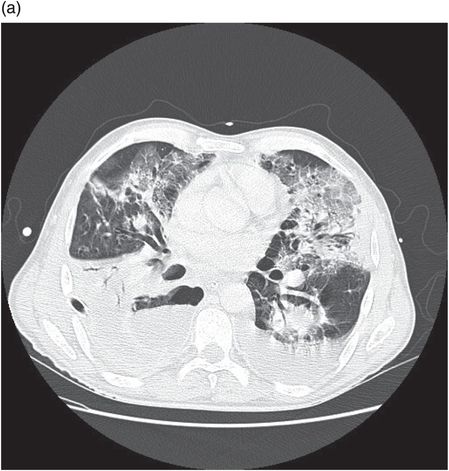
Chest CT showing lung abscess and empyema. Klebsiella species were cultured from pleural drain fluid.
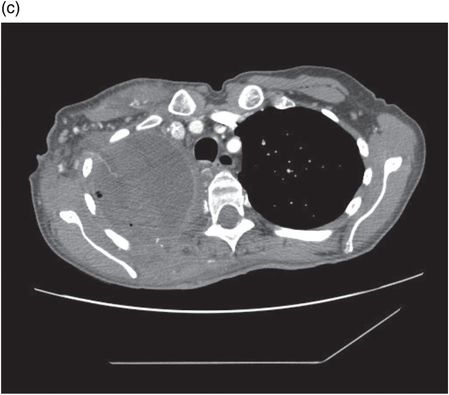
Chest CT showing pectoralis muscle flap utilized to obliterate residual pleural space following thoracotomy and drainage in same patient.
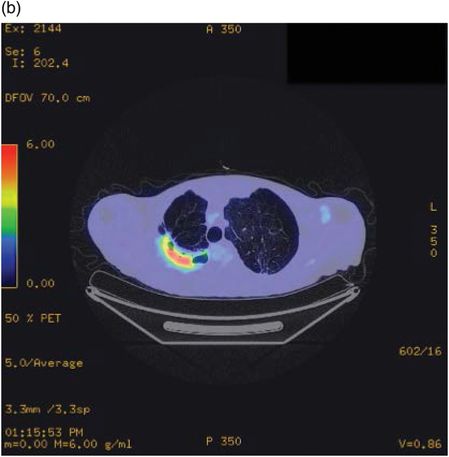
Fludeoxyglucose positron emission tomography (FDGPET) demonstrating FDG avidity of pleural aspergilloma prior to resection in a patient with significant emphysema.
Empyema in children
In children, empyemas almost always occur as a complication of respiratory tract infection. The goals of management of empyema in children are similar to those in adults, which are to eliminate the empyema, re-expand trapped lung, restore mobility of chest wall and diaphragm, return respiratory function to normal, eliminate complications of chronicity and reduce length of hospital stay. Two randomized, controlled trials have found that there is no difference in the length of hospital stay and success rate between VATS and chest tube drainage with fibrinolytics[56,57]. However, when patients were randomized to either VATS or chest tube, with fibrinolytics as indicated, Kurt and colleagues found that patients treated with VATS had significantly shorter hospital stay[58]. A systematic review which included 3418 patients from 54 different studies concluded that VATS significantly improved mortality (0 vs 3.3%), re-intervention rate (2.5 vs 23.5%), duration of chest drains (4.4 vs 10.6 days) and length of hospital stay (10.8 vs 20 days)[59].
Landmark and important publications
Lung abscess
Aetiology
Lung abscess is defined as a localized area of suppuration occurring in association with cavitation within the lung. Abscess formation may occur primarily as a consequence of initial necrotizing infection or result from secondary causes including infection of a pre-existing cavity, bronchial obstruction due to foreign body or tumour or due to septic emboli. Cavitating conditions of the lung including pulmonary infarction, sarcoid and Wegener’s granulomatous; all predispose to lung abscess.
The risk of developing necrotizing infection is increased in the immunocompromised patient and by factors that increase the risk of aspiration such as altered mental state particularly alcohol misuse, vocal cord paralysis, poor dental hygiene and oesphageal pathology such as diverticulae or malignancy. Poor dental hygiene is so commonly associated that suspected lung abscess in the edentulous should cause reconsideration of the diagnosis and further efforts to exclude underlying malignancy should be made.
Such pre-disposing factors are identified in the majority of patients diagnosed with lung abscess. In a series of 259 patients presenting with lung abscess, overall poor health was seen in over 97%, 82.5% had associated dental disease, 78.6% reported having lost consciousness at least once and 70.2% described alcohol misuse[60].
Pathophysiology/microbiology
The process has arbitrarily been divided into acute and chronic phases based on a 6-week time period; however, in practice, the onset is insidious, and these phases are not therefore useful in the diagnosis and management of the condition.
The classic studies of lung abscess were performed by David Smith at Duke in the 1920s[61]. He noted the similarity between bacteria in the mouth and those identified in the walls of the lung abscesses at post mortem, leading him to postulate that aspiration was the mechanism of infection, which he confirmed by an inoculation model of lung abscess.
Lord Brock later radiologically demonstrated that the segmental localization we observe in lung abscess is associated with aspiration by using iodized oil in patients in the recumbent position. In the supine position, aspiration preferentially targeted the posterior segment of the right upper lobe and the apical segment of the lower lobes[62].
Anaerobic bacteria accounted for 60–80% of cases of lung abscess, with the predominant isolates being mixed Peptostreptococcus, Fusobacterium, Prevotella and Bacteroides species[60,63]. In primary necrotizing pneumonia, Staphylococcus aureus, Streptococcus pneumoniae and gram-negative organisms especially Klebsiella are more commonly seen to predominate rather than mixed cultures[64]. Other culprit pathogens include Haemophilus influenzae, Actinomyces species, Aspergillus, Cryptococcus, Histoplasma, Blastomyces and Coccidioides.
Enatamoeba histolytica results in lung abscess following haematogenous spread and is almost always associated with empyema. It is classically associated with anchovy paste sputum due to expectoration of amoebae and liver tissue. Metronidazole is the antibiotic of choice.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree