CHAPTER 110 Surgical Approaches, Cardiopulmonary Bypass, and Mechanical Circulatory Support in Children
For infants and children, mechanical support of the circulation has important roles in providing short-term circulatory support during reversible myocardial failure, in providing cardiopulmonary support before and after cardiac surgery, and in providing longer-term support as a potential bridge to cardiac transplantation. Modalities of support include extracorporeal membrane oxygenation (ECMO), intra-aortic balloon pump (IABP) counterpulsation, and ventricular assist devices (VADs). Although a variety of assist devices are available for adult-size patients, the need for miniaturization has delayed their application in children; ECMO is therefore still the most common form of mechanical circulatory support for pediatric patients. ECMO was first introduced as respiratory support in pediatric patients with severe lung disease, and institutions with an established ECMO program have been able to transition this methodology to provide biventricular support and oxygenation in pediatric patients with a failing circulation.1–3 There are no established guidelines for the indications or management of cardiac ECMO support, and there is considerable inter-institutional variability with respect to use and outcomes, depending on experience and philosophy. VADs offer the potential for both short- and longer-term support of circulation in patients who do not have concurrent pulmonary parenchymal or vascular disease, and there is increasing experience in developing this form of support as an effective longer-term bridge to transplantation.
EXTRACORPOREAL MEMBRANE OXYGENATION
Overview of Uses
The use of ECMO to support children with impaired gas exchange as a result of acute respiratory failure is now an accepted and successful therapy, particularly in neonates with a variety of parenchymal and vascular lung diseases (e.g., meconium aspiration, respiratory distress syndrome, diaphragmatic hernia, persistent hypertension of the newborn).3 The positive effect of ECMO on outcome in these patients depends mainly on the early diagnosis of severe pulmonary failure, the prompt institution of ECMO, and the reversible nature of the pulmonary dysfunction. However, the advent of other therapies, such as high-frequency oscillatory ventilation, surfactant therapy, permissive hypercapnia, and inhaled nitric oxide, has led to a reduction in the need for ECMO in neonates.4–6 According to the cumulative data reported by the Extracorporeal Life Support Organization (ELSO) Registry, 76% of all neonates who have been placed on ECMO for respiratory support have survived to discharge from hospital.4 The outcome for older patients is considerably lower, with the reported cumulative survival for pediatric and adult patients placed on ECMO for respiratory support being approximately 50%.
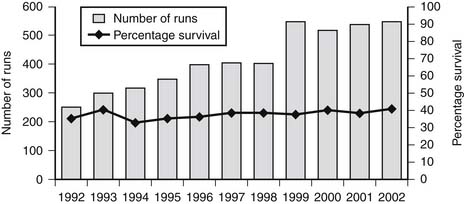
Although ECMO for respiratory use has decreased, the past decade has seen a steady increase in the number of patients and institutions using ECMO to support a failing circulation, mostly after congenital cardiac surgery or as a bridge to transplantation.4,7–18 Cardiac ECMO has also been deployed rapidly during resuscitation from cardiac arrest, but this is use is more controversial.7,9,10,19–21 Despite the increased enthusiasm for ECMO support of the circulation, the survival to discharge as reported by the ELSO (38% for neonates and 39% for pediatric patients) has not increased over the past decade (Fig. 110-1) and has lagged considerably compared with the experience with respiratory ECMO (Table 110-1).4 The majority of cardiac patients are placed on ECMO after surgery. Adverse outcomes after cardiac ECMO are primarily related to irreversible underlying cardiac disease and to the presence of significant end-organ injury. Recovery from severe myocardial dysfunction while on mechanical support does occur, provided the myocardium has sustained a transient and reversible injury. ECMO facilitates ventricular recovery by reducing myocardial wall tension, increasing coronary perfusion pressure, and maintaining systemic perfusion with oxygenated blood.
Table 110–1 Neonatal Respiratory and Cardiac ECMO: Differences in Survival Reported by the ELSO Registry for Specific Diagnostic Groups
| Diagnosis | Survival (%) |
|---|---|
| Meconium aspiration syndrome | 94 |
| Primary pulmonary hypertension | 78 |
| Sepsis | 75 |
| Air leak syndrome | 74 |
| Congenital diaphragmatic hernia | 51 |
| Cardiac disease | 38 |
ECMO, extracorporeal membrane oxygenation.
From Extracorporeal Life Support Organization (ELSO): International Summary, Jan 2008.
It also is important to appreciate the differences between the ECMO circuit and management, and the routine cardiopulmonary bypass (CPB) used during cardiac surgery. The ECMO circuit is a closed circuit. It has limited ability to handle any air in the venous limb of the circuit, and careful de-airing of both the arterial and venous cannulas is essential when connecting to the ECMO circuit. The average duration for cardiac ECMO runs reported to ELSO over the past 15 years has increased slightly from approximately 4 to 5 days to about 5 to 7 days; the longest reported run has been 62 days.4 These data emphasize that ECMO should be viewed only as a relatively short term support of the circulation, and beyond 7 days the chances of successful decannulation and survival decrease substantially. These data also support the need to develop longer-term mechanical support devices, particularly as a bridge to transplantation.
The significant time limitation associated with cardiac ECMO means that, ideally, only patients with known reversible cardiac disease should be considered candidates for cardiac ECMO, but this is often not possible when a rapid decision needs to be made to place a patient on ECMO because of cardiac arrest or a severe low cardiac output state. In general, institutions with an efficient and well-established ECMO service are more likely to use this form of support for the failing circulation, and surgeon bias, case type, surgical techniques, and CPB management are additional confounding factors that make comparisons among institutions difficult.22 Although it could be argued that ECMO should be readily available in any center undertaking complex congenital cardiac surgery, establishing a structured and coordinated team approach to cannulation is a key step for any ECMO service.10 In our experience at Children’s Hospital Boston, the introduction of a dedicated cardiac ECMO program and development of a rapid response system for its use during active resuscitation has contributed to an increase in the survival-to-discharge rate for ECMO circulatory support, from 45% in 1995 to 59% in 2002, regardless of the diagnosis or indication for cardiac support.20
Indications
According to the ELSO Registry report of outcomes based on broad diagnostic categories, patients placed on ECMO because of complications related to fulminant myocarditis have the highest survival rate (Table 110-2), although it lags behind the successful outcomes achieved with ECMO for respiratory support in neonates. The survival from cardiac ECMO to hospital discharge according to selected congenital defects is shown in Table 110-3. The heterogeneity of diagnosis and procedures for which ECMO has been used is well demonstrated, but the survival rate is poor across all groups, and there is no specific cardiac procedure or diagnostic group for which ECMO is a proved therapy. Rather than trying to determine indications for cardiac ECMO according to specific diagnoses or procedures, the indications can be examined in five broad categories: preoperative resuscitation; inability to wean from cardiopulmonary bypass; postcardiotomy; cardiomyopathy, myocarditis, and bridge to transplantation; and after in-hospital cardiac arrest and cardiopulmonary resuscitation (CPR).
Table 110–2 Survival for Cardiac ECMO Runs Based on Broad Diagnostic Categories
| Diagnosis | Survival (%) |
|---|---|
| Congenital cardiac defect | 43 |
| Cardiac arrest | 38 |
| Cardiogenic shock | 40 |
| Cardiomyopathy | 57 |
| Myocarditis | 64 |
ECMO, extracorporeal membrane oxygenation.
From Extracorporeal Life Support Organization (ELSO): International Summary, January 2008.
Table 110–3 Survival for Selected Congenital Cardiac Defects by Age and Diagnosis
| Diagnosis | Neonates (%) | Infants (%) |
|---|---|---|
| Left-right shunt | 34 | 40 |
| Left obstruction | 29 | 38 |
| Hypoplastic left heart syndrome | 27 | 36 |
| Right obstruction | 42 | 44 |
| Cyanosis (with increased pulmonary blood flow) | 31 | 49 |
| Total anomalous pulmonary venous return | 43 | 36 |
| Cyanosis (with decreased pulmonary blood flow) | 39 | 39 |
| Other | 42 | 47 |
From Extracorporeal Life Support Organization (ELSO): International Summary, January 2008.
Preoperative Resuscitation
ECMO may be beneficial for critically ill patients before cardiac surgery, enabling preoperative stabilization, optimization, and prevention of end-organ dysfunction before repair. These patients represent a small group (usually newborns), and indications include severely low cardiac output states (e.g., critical aortic stenosis), pulmonary hypertension (e.g., obstructed total anomalous pulmonary venous drainage), and severe hypoxemia (e.g., transposition of the great arteries and pulmonary hypertension).22–24
Inability to Wean from Cardiopulmonary Bypass
The reported survival of patients placed on ECMO because they were unable to wean directly from CPB in the operating room (i.e., without any period of stability off CPB) is poor.7,8,15,22 Issues such as primary myocardial dysfunction, pulmonary hypertension, severe hypoxemia, and refractory dysrhythmias are recognized as major factors in determining successful outcome, but unrecognized residual or irreparable defects are also important.8 These defects must be searched for in the operating room, preferably in combination with echocardiography and the careful measurement of oxygen saturations and intracardiac pressures. Ideally, only children with potentially reversible myocardial injury who cannot be weaned from CPB should be considered candidates for ECMO, but this may be extremely difficult to determine in the operating room immediately after cardiac surgery. Considerations include preoperative condition, intraoperative course, and the likelihood of being a transplant candidate. Severe hemorrhage is a major problem in the transition from the CPB circuit to the ECMO circuit. Although a lower activated clotting time (ACT) (160 to 180 seconds) can be used, small doses of protamine are often necessary to assist with the initial control of bleeding. We usually administer protamine in 1-mg/kg increments until a target ACT of 180 seconds is achieved. Infusions of antifibrinolytic drugs such as tranexamic acid (bolus of 100 mg/kg, followed by infusion at 10 mg/kg/hr), or ε-aminocaproic acid (bolus of 100 mg/kg, followed by infusion at 30 mg/kg/hr) should be considered. Exploration of the chest may be necessary, particularly if the bleeding persists at a rate greater than 10 mL/kg/hr and if problems with ECMO flow are encountered because of decreased venous cannula drainage from a tamponade-like effect. The large transfusion requirement may place a considerable burden on the supply of donor blood products. As an alternative, it is possible to connect a chest tube to cell-saver tubing to enable blood to be collected in the cell-saver reservoir and subsequently spun for retransfusion.
When a patient is placed on ECMO in the operating room, discussions with the patient’s family must be clear and direct. Recovery of myocardial function should be expected within 2 to 3 days,25 and if this is not evident, either listing for cardiac transplantation, if appropriate, or withdrawal from support must be considered.
After Cardiotomy
ECMO is an effective therapeutic option for infants and children who have had a period of relative stability after successful termination of CPB, and when significant residual cardiac defects are excluded. Myocardial or respiratory failure causing a low cardiac output state, hypoxemia, or pulmonary hypertension and cardiac arrest are the major indications in this group. This is a large group of patients, and reported survival rates are as high as 60% to 70%, provided ECMO is instituted rapidly and effectively.7–9,15,22,26
Cardiomyopathy, Myocarditis, and Bridge to Transplantation
Patients who have acute fulminant myocarditis can be managed successfully with ECMO.4,27 Some of these patients may be candidates for VADs, even though their disease is usually biventricular, but ECMO is often preferable. Patients with fulminant myocarditis may arrive at the hospital undergoing full cardiac arrest, but more commonly they are in shock from an extremely low cardiac output state or they have hemodynamically significant dysrhythmias, including ventricular tachycardia or heart block. The heart is usually distended and is contracting very poorly. Prompt institution of ECMO may allow sufficient resuscitation and stabilization to prevent end-organ injury and enable the myocardium to rest while awaiting potential recovery. After ECMO is instituted, the heart must be fully decompressed, and urgent atrial septostomy or left atrial vent placement may be necessary.28 The heart may not begin to eject for the first 24 to 36 hours after ECMO is started, although recovery of electrical activity within the first few hours should be expected. If recovery of ventricular ejection is not evident within 2 to 3 days, ECMO can be continued either as a bridge to heart transplantation or as a bridge to alternative longer-term support with a VAD, if feasible.13,25,29–36 ECMO should be viewed as a short-term bridge to transplantation because of the limited donor availability and the time-related risks for complications, such as infection, bleeding, end-organ impairment, problems resulting from immobilization, and difficulties in maintaining adequate nutrition.31,37 In our experience at Children’s Hospital Boston, the median time spent on ECMO awaiting heart transplantation is currently 140 hours (range, 26 to 556 hours), but only 50% of our listed patients have been effectively bridged. In a small number of older and larger children, ECMO has been used to initially resuscitate the circulation and end-organs, and if a donor heart has not become available by day 6 or 7 of ECMO, we have successfully transitioned from ECMO to longer-term VAD.
ECMO also has been used to effectively support the failing heart after transplantation. This may be necessary immediately after transplantation because of graft failure, usually in the setting of pulmonary hypertension and acute right ventricle failure of the donor heart. ECMO also is effective in ingsupport the heart during periods of acute rejection.33 The inflammation and myocardial edema are similar to that seen with fulminant myocarditis, and they lead to a similar spectrum of clinical features. ECMO allows the transplanted heart to decompress with decreased wall tension while antirejection therapy is increased. In our experience, survival to discharge for this indication is currently 64%, and the median duration of ECMO support is 4 days.
After In-Hospital Cardiac Arrest and CPR
Survival and outcome after in-hospital resuscitation of pediatric patients after a cardiac arrest continues to be extremely poor.38–43 Even in a highly monitored and resource-intensive area such as a pediatric intensive care unit, the survival rate after cardiac arrest has been reported to be only between 9% and 31%. The duration of cardiac arrest and resuscitation is also an important determinant of subsequent outcome, and a number of reports have noted a critical threshold of approximately 15 minutes.38,39 However, there are reports of successful use of ECMO to support children after prolonged periods of cardiac arrest that have been unresponsive to closed or open cardiac massage and all other usual interventions.7,19,20 Again, it is important to emphasize that the underlying lesion, in conjunction with the effectiveness of CPR while instituting ECMO, is a major determinant of outcome when mechanical support is used in this setting. Although the exact place of ECMO in the CPR algorithm remains ill defined, patients with a witnessed arrest and rapid institution of effective CPR, and who have no apparent recovery of cardiac function within 10 to 15 minutes of initiating resuscitation and no contraindications, may be suitable candidates for ECMO.
Determining the relative contraindications to ECMO support during active resuscitation attempts can be difficult (Table 110-4). Preferably, discussions regarding the use of ECMO in certain patients should be undertaken before an event occurs, although clearly this is not always possible. We have been able to successfully use our rapid-response ECMO system during CPR in patients with acquired and structural heart disease, and in patients with double- or single-ventricle defects. In the latter group are neonates who have had a sudden, reversible event, such as acute thrombosis and obstruction to a systemic-to-pulmonary artery shunt after a Norwood procedure, and they have been readily resuscitated with ECMO. On the other hand, patients with cavopulmonary corrections (i.e., Fontan or bidirectional Glenn anastomosis) have been difficult to resuscitate using ECMO, in part because of limitations with cannulation, and an inability to maintain adequate systemic oxygen delivery and avoid cerebral venous hypertension during CPR with chest compressions. Although we have used ECMO in the resuscitation of patients with pulmonary hypertension and patients with systemic outflow obstruction, the severe limitation to cardiac output and oxygenations during CPR in these patients has meant that their overall outcomes on ECMO have been poor because of the development of severe end-organ injury.
Table 110–4 ECMO Support during Active Cardiopulmonary Resuscitation
| Resuscitation Event | Considerations |
|---|---|
| Indications | |
Event witnessed and monitored (e.g., tamponade, arrhythmia, systemic to pulmonary artery shunt, obstruction) | |
| Absolute Contraindications | |
| Relative Contraindications | |
ALS, advanced life support; BLS, basic life support; CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; OR, operating room.
Historically, successes using ECMO during active resuscitation and chest compressions (E-CPR) have been reported for a small number of series.7,19–21,44 A recent subanalysis of the ELSO Registry E-CPR data reports a 42% overall survival to discharge for patients placed on ECMO in this circumstance.4 To avoid significant delays, a rapid response ECMO system has been established at some institutions.20,21 The success of a rapid response system depends on a multidisciplinary approach, with equipment being immediately available, and the personnel with assigned roles being in-house, including cardiac surgery and intensive care fellows, respiratory and ECMO specialists, and trained nursing staff. At Children’s Hospital Boston, a vacuum and CO2-primed circuit using a roller pump and a 0.8- to 1.5-m2 membrane oxygenator is available at all times and is suitable for children weighing up to approximately 15 kg. Even in older children, this circuit initially provides sufficient flow for resuscitation, stabilization, and (it is hoped) prevention of end-organ damage, until a larger oxygenator can be spliced into the circuit. Generally, however, for older children and adults, a new circuit with a hollow-fiber membrane is used, which takes little time to de-air and can be established within 15 minutes. Once the patient is stable on ECMO, the hollow-fiber membrane can be exchanged for a conventional membrane for longer-term support as necessary. An alternative rapid response system has been described, which uses a heparin-coated circuit, centrifugal pump, and a hollow-fiber membrane with a priming volume of only 250 mL and a priming time of only 5 minutes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree