CHAPTER 68 Surgery of the Aortic Arch
The history of cardioaortic surgery has been replete with new techniques for ascending and aortic arch repairs since 1956, when Denton Cooley and Michael DeBakey replaced the ascending aorta with a homograft using cardiac bypass.1–63 In 1957, DeBakey and colleagues12 first described aortic arch replacement using antegrade brain perfusion. Before this, one of the few successful ascending aortic repairs was reported in 1932 by Blalock, who repaired a stab wound of the ascending aorta caused by an ice pick.47 By removing the occluding clot, Blalock noted the “bright red blood that shot over the screen at the head of the table on the anesthetist’s clothes.” Using only nitrous oxide and oxygen for anesthesia in a patient with cardiac tamponade most likely contributed to the hypertension and description of the procedure.
With the advent of cardiopulmonary bypass, many technical problems of aortic arch surgery were largely overcome. In 1950, Bigelow first published the results of his experiments in dogs using deep hypothermia for cardiovascular operations without cardiopulmonary bypass.47 Nonetheless, it was not until 1963 that Barnard and Schrire2 combined deep hypothermia with circulatory arrest and cardiopulmonary bypass for aortic arch operations and dissection. In 1964, Borst reported using deep hypothermia and circulatory arrest to repair an arteriovenous fistula between the innominate vein and the aorta caused by shrapnel.47 The routine use of deep hypothermia and circulatory arrest did not, however, become popular until 1975, when Griepp reported a series of aortic arch replacements using deep hypothermia and circulatory arrest.47 In 1983, Borst and colleagues3,4 and others22 reported replacing the aortic arch and leaving a tube graft lying free in the descending aorta, which they called the elephant trunk technique. In 1990, Crawford and Svensson47,57 reported replacement of the entire aorta as a staged procedure using a modified elephant trunk technique. The modification of inverting the graft into the descending aorta while sewing the distal anastomosis resulted in a more secure anastomosis with less risk of bleeding or rupture.57 Subsequently, in 1993, Svensson and colleagues47 successfully replaced the entire aorta from the aortic valve to the aortic bifurcation during a single operation using a combined mediastinal and thoracoabdominal incision with deep hypothermia and circulatory arrest.12
Currently, aortic arch surgery has become relatively common and safe with a mortality risk of 2% and a stroke risk of 2%.55 In 2007, we performed 998 aorta operations, 541 of them on the ascending aorta and aortic arch. Our approach is discussed later. In this chapter, we will discuss the potential etiologies of aortic arch aneurysms, the association with some pathologic entities, diagnostic workup, brain protection, perfusion methods, different operative approaches, and outcomes after aortic arch surgery.
DEFINITIONS AND CLASSIFICATION
Anatomically, the aortic arch is defined as the segment of aorta between a line at a right angle proximal to the innominate artery origin and extending to a line drawn at a right angle distal to the origin of the left subclavian artery. Aneurysms are irreversible dilations of the aorta exceeding the normal diameter for the age and height of the patient.47 The exact size at which the aorta is labeled “aneurysmal” varies. Definitions vary from 1.5 times to twice the normal diameter of the aorta. For patients with Marfan syndrome, we suggest that when the cross-sectional area (in square centimeters) divided by the patient’s height (in meters) exceeds a ratio of 10, it should be considered significant and an indication that the patient requires surgical repair.50 Thus, in some respect, the definition of an aneurysm is not absolute but rather refers to the significant dilation of the aorta.
Aneurysms can be divided further into aortic aneurysms without penetration through the aortic adventitia (true aneurysms) and those that penetrate through the adventitia and are contained by the surrounding tissue, which prevents exsanguination of the patient (false aneurysms).47 In addition, aneurysms are classified according to their likely etiology: medial degenerative aneurysms (typically showing loss of elastic tissue); those related to aortic dissection; other disorders of connective tissue, particularly loss of collagen as in Ehlers-Danlos syndrome or loss of elastic tissue as in Marfan syndrome; those associated with blunt trauma; aortitis; primary aortic infections or after previous cardiovascular surgery, especially graft infection in the ascending aorta; and congenital abnormalities.47
We prefer the term medial degenerative aneurysms to atherosclerotic aneurysms simply because not all medial degenerative aneurysms have atherosclerosis.47 Furthermore, atherosclerosis does not necessarily appear to be the sole etiologic factor in the development of medial degenerative aneurysms. It appears that atheroma formation, fibrosis, and calcification are results of degeneration that follow the primary injurious event that caused the aneurysm.
Aneurysms can be either fusiform, showing uniform dilation, or saccular in appearance.47 The three most common sites for saccular aneurysms are on the lesser curve of the aortic arch, on the descending aorta, and opposite the visceral vessels.47 The saccular aneurysms in the aortic arch are usually related to penetrating ulcers, often with localized dissection or mycotic aneurysm formation.47 Fusiform aneurysms of the aortic arch are typically associated with dilation of the ascending aorta, particularly when associated with inflammatory aortitis. Medial degenerative aneurysms of the root are known as annuloaortic ectasia, a term coined by Cooley.47 This results in a flasklike or hourglass appearance of the aortic root (Erdheim deformity), which is also associated, in particular, with Marfan syndrome. For example, in patients with Marfan syndrome, initiation of aortic root dilation results early in the annuloaortic ectasia stage, and, if it is not treated, there is subsequent development of aortic root, ascending aorta, and aortic arch aneurysm formation. In fact, at this late stage, aortic dissection often precedes aortic arch aneurysm formation.
The human artery consists of five distinct layers. The first or innermost layer (the endothelial layer, which lies on a basement membrane) is known as the tunica intima. Between the tunica intima and the tunica media is a fenestrated sheath of elastic fibers known as the internal elastic lamina. The tunica media has several layers of elastic-tissue lamellae arranged concentrically along the length of the aorta, and it forms the bulk of the aortic wall. The amount of elastic tissue decreases in amount from the sinotubular ridge as the aorta progresses down to the aortic bifurcation. In the tunica media lie smooth muscle cells and the ground substance of the aorta. The latter consists of proteoglycans. The outer third of the tunica media receives its nutrition from the vaso vasorum, lymphatics, and nerves. On the outside of the tunica media is the external elastic lamina, which separates the media from the adventitia. The tunica adventitia consists of strong, tough layers of collagen and elastic fibers. Because of its strength, this is the critical layer wherein the surgeon must suture the graft placement.47
There is no clear, systematic classification of aortic pathology in the literature, and different pathologists have coined a variety of terms. We favor a definition of aortic pathology based on hematoxylin and eosin (H&E) findings and elastic tissue stains. Thus, medial degenerative disease is defined as a loss of elastic fibers, and medial necrosis as a loss of smooth muscle cells. The presence of atherosclerosis superimposed on degenerative disease is described as atherosclerotic with or without calcification. Atheroma is also frequently superimposed. Inflammatory disease is diagnosed when there is evidence of chronic inflammatory cell infiltrates. The intima and adventitia may also show various degrees of hyperplasia.47
ETIOLOGIC AND PREDISPOSING FACTORS
Congenital Aneurysms
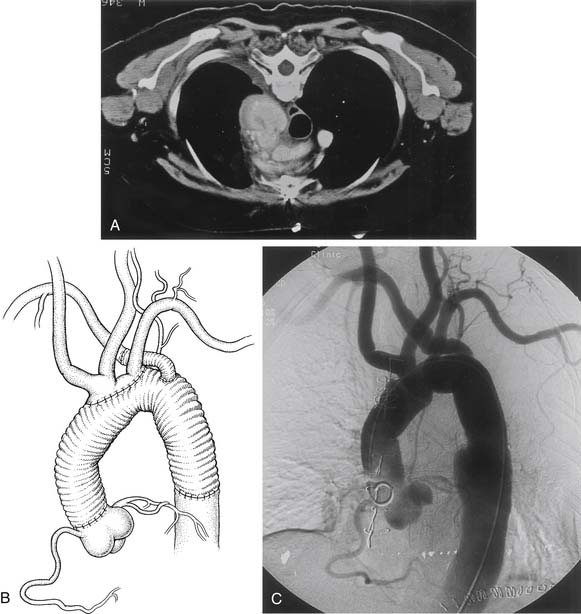
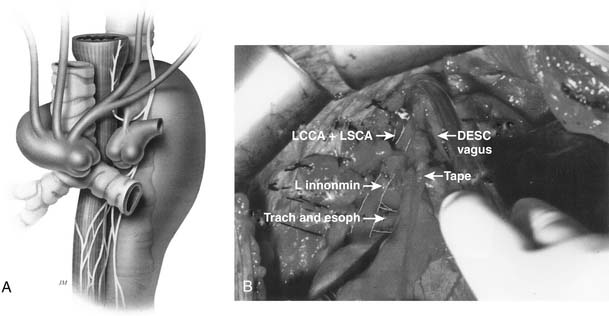
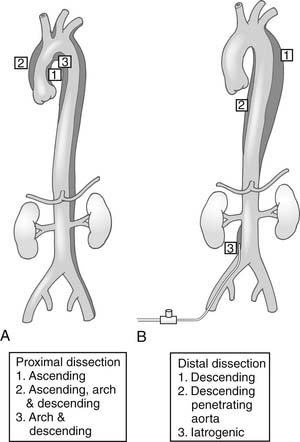
Congenital aneurysms of the aortic arch are extremely rare, although they may be associated with aberrant right subclavian arteries from a Kommerell’s diverticulum situated in either the distal aortic arch or the proximal descending aorta (Figs. 68-1 and 68-2).47 Similarly, aneurysms can be associated with one of two types of right-sided congenital arches. For the Felson and Palayew type I right-sided arches, a vascular ring encircles and compresses the esophagus and trachea (Fig. 68-3).36 The distal arch and descending aorta may be aneurysmal. In patients with type II right-sided arches (Fig. 68-4), the anatomy is basically a mirror image of an aberrant right subclavian artery with a Kommerell’s diverticulum. Thus, an aberrant left subclavian artery comes off the right-sided descending aorta.36 More frequently, however, the aortic arch, in association with these two varieties, is hypoplastic unless the hypoplasia is severe enough to cause aortic stenosis, and prestenosis or poststenosis aneurysm formation occurs. Coarctation of the aorta is often associated with a bicuspid valve and an ascending aortic aneurysm, but, if left untreated, it may sometimes be associated with aortic arch aneurysm formation. A history of patent ductus arteriosus or ventricular septal defect may also be noted. More rarely, adult patients who have been previously operated on for an interrupted aortic arch may present with ascending and aortic arch aneurysms.47
Medial Degenerative Aneurysms
Medial degenerative aneurysms typically occur in older adult patients who were long-term smokers or have a long history of hypertension. If the smoking history is severe and there is presence of chronic obstructive pulmonary disease (COPD), extensive atheroma formation may also be found in the aneurysms. These types of aneurysms, with extensive atheroma and atherosclerosis formation, typically involve not only the ascending aorta and the aortic arch but also the descending and thoracoabdominal aorta. Coronary artery disease and carotid artery disease are also common associations. When the aorta is inspected, small ulcers are often observed, and these can later form penetrating ulcers that result in dissection in the medial adventitial plane or false aneurysm formation if the adventitia is penetrated.47 Less typically, medial degenerative aneurysms are associated with systemic inflammatory disease and various types of arthritis or vasculitis (see later).
Aortic Dissection
There is a difference between the DeBakey and Stanford classifications of aortic dissection, and there are also different classes of dissection51 (Fig. 68-5) regarding replacement of the aortic arch. The class of intimal tear also influences the aortic arch repair technique.51
Mycotic Aneurysms
For reasons not entirely clear, true primary mycotic aneurysms of the aorta, as described by Svensson and Crawford,47 have a tendency to occur either on the lesser curve of the aortic arch, with a variable extent of involvement, or opposite the visceral vessels in the abdomen. It is unclear whether this is related to the structure of the aorta or to the flow patterns that result in turbulence in these areas opposite the branches of the aorta. The typical infective organisms are Escherichia coli, staphylococci, Salmonella, and streptococci including Streptococcus pneumoniae. Other strains, however, may sometimes be detected. Some of these may be related to atherosclerotic ulcers, which initially act as a nidus for subsequent infection. Mycotic aneurysms frequently penetrate through the aortic arch wall, resulting in false aneurysms or free rupture. Osler called these aneurysms mycotic because the gray, slimy lining reminded him of fungal growth. Fungal infections in the vessel walls are, on the whole, very rare, except in patients who have had graft-related infections.47
Vasculitis and Aortitis
Inflammatory infiltrates of the aorta are not infrequent. In a prospective examination of histologic specimens by both H&E and elastic-tissue stains, inflammatory infiltrates were found in many of the specimens.53 Although these inflammatory infiltrates consist of various types of leukocytes, often associated with the diseases listed later, an associated systemic illness may be absent. This suggests that the original cause of the aortic injury was unrecognized, subsequently appearing as a medial degenerative aneurysm, with formation of atherosclerosis and calcification. Previous chest radiation for Hodgkin’s disease or breast malignancies may also be noted in the history. Radiation-induced vasculitis is associated with severe calcification and a porcelain aorta. A stiff left ventricle, scarred right ventricle, and fixed cardiac output increase the risk of surgery.
Inflammatory systemic diseases may result in aneurysms. For example, the development of aortitis is commonly associated with Takayasu’s disease (nonspecific aortoarteritis) (Fig. 68-6); giant cell arteritis (Horton’s disease); temporal arteritis; polymyalgia rheumatica; Behçet’s, Buerger’s, Logan’s, Sjögren’s, Reiter’s, or Kawasaki disease; relapsing polychondritis; systemic lupus erythematosus (often mycotic); rheumatoid arthritis; sarcoid and ankylosing spondylitis; osteoarthritis of unknown origin; ulcerative colitis; and potentially autoimmune diseases of the thyroid, such as Hashimoto’s.
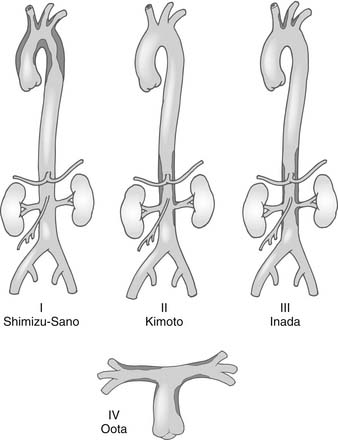
Figure 68–6 Classification of Takayasu’s aortitis. Subtypes and their extents are depicted. PA, pulmonary artery.
(From Svensson LG, Crawford ES. Cardiovascular and vascular diseases of the aorta. Philadelphia: WB Saunders; 1997, p. 42-83.)
Trauma
Ten percent of traumatic lesions of the aorta occur in the aortic arch. The remaining 90% occur in other segments that will be discussed in Chapters 69 and 75. The aneurysms are most often related to tears at the hinge point of the aorta during acceleration or deceleration injuries. Thus, the usual sites of primary tears are either the origin of the innominate artery or the subclavian artery (70% to 80%), although the origin of the common carotid artery may also be involved.47 Although blunt trauma is the most common cause in the United States, traumatic arch injuries are more frequently caused by penetrating injuries from shrapnel or bullets or knife injuries in the Third World. With penetrating lesions, involvement of the trachea, esophagus, venous system, nerves, and vertebral column significantly complicates management.
Tumors of the Arch
Tumors of the aortic arch are extremely rare, but when they occur they are usually found distal to the subclavian artery. Very rarely have tumors been related to prosthetic grafts.47
Reoperations
The first scenario involves a patient who earlier underwent acute dissection repair and subsequently presents with an aortic arch aneurysm because the aorta, weakened from the dissection, has dilated over the course of time. This situation occurs commonly in patients with Marfan syndrome. It is preferable to repair only the ascending aorta at the time of the acute dissection repair,46,47 first because the first priority is to save the patient’s life, and second because trying to repair the aortic arch simultaneously carries a much higher mortality risk.46,47 Furthermore, the risk of a patient’s requiring another operation after acute dissection repair is, in most cases, small.46,47 It is, however, important that the initial acute dissection repair should be performed with circulatory arrest unless the dissection is a DeBakey type II. This is so that the inside of the aortic arch can be inspected and a more complete hemiarch repair can be performed at the initial operation. A tear in the aortic arch can often be repaired with pledgeted 4-0 Prolene running sutures.46,47 The use of biological glues may also increase the risk of recurrent aneurysms.
An uncommon group of patients who require reoperations are those who form aneurysms at either the origin of the greater vessels, most typically the innominate artery, or at the site where a Carrel patch of the aorta was used for reattachment of the greater vessels. The site becomes aneurysmal and enlarges. This latter scenario should be watched for particularly in patients with Marfan, Loeys-Dietz, or Ehlers-Danlos syndrome, especially if they have suffered aortic dissection and the aorta is fragile.43,46,47
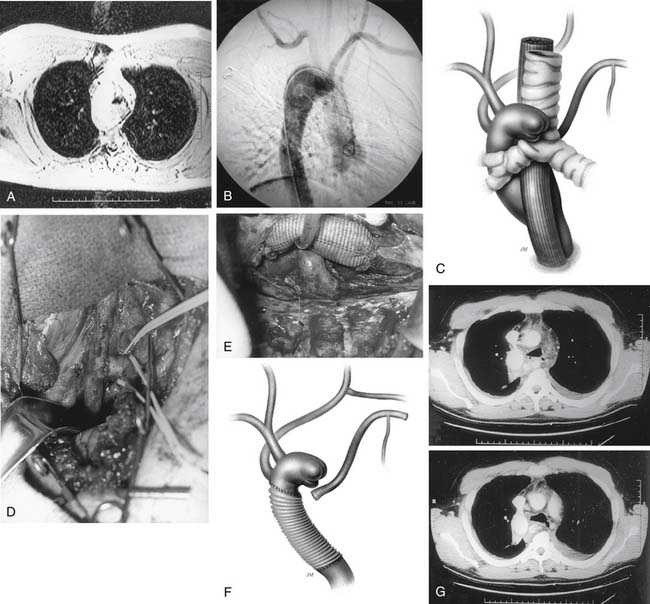
Although aortic graft infections are rare in our experience, we are often referred such cases. They require extensive débridement and repair, usually with homografts (allografts). If there is a cavity, omental or muscle flaps are used to fill the space (Fig. 68-7). Patients are kept on lifelong antibiotics or, for fungal infections, fungal antimicrobials, particularly the newer, less toxic form of amphotericin B.
Syndromes Associated with Aortic Arch Pathology
Aneurysms may form in association with genetic diseases such as Marfan syndrome, Ehlers-Danlos syndrome, Erdheim’s syndrome (annuloaortic ectasia), Noonan’s syndrome, hereditary polycystic kidney disease, osteogenesis imperfecta, and Turner’s syndrome.47
HISTORY AND PHYSICAL EXAMINATION
When patients present with a potential aortic arch aneurysm, neither questioning about symptoms nor physical examination is particularly informative. During the questioning, however, any history of aortic valvular disease associated with aortic root aneurysms needs to be sought, along with any history of neurologic or neurocognitive events. Computed tomography (CT) scans or magnetic resonance imaging (MRI) studies prior to aortic arch surgery have shown that between 40% and 60% of patients have evidence of brain injury. In a prospective randomized study, 38% of our patients, excluding those who had strokes with residual deficits and those older than 75 years, had a neurocognitive deficit prior to undergoing aortic arch surgery.48,54
If the aortic arch aneurysms are particularly large, hoarseness may develop, particularly if the distal aortic arch is enlarged. The reason is that the left recurrent nerve wraps around the distal aortic arch at the ligamentum arteriosum and can become stretched by the aneurysm. Dysphagia may also occur, but this is more frequent with congenital lesions (see Fig. 68-4). In 1794, Bayford called this “lusoria,” referring to the Latin term lusus naturae meaning “a freak of nature.” This is often described as dysphagia lusoria or arteria lusoria. Dyspnea can also be a symptom when the pulmonary artery or left bronchus is constricted.47
Patients may complain of occasional mid-scapular pain if the aneurysms are large, although this is associated more with enlarged descending or thoracoabdominal aorta aneurysms. With aortic dissection or a false aneurysm related to a penetrating ulcer or mycotic aneurysm, severe chest pain may be present. This may be anterior or posterior chest pain with extension into the neck (see Chapters 70 and 71).
Preoperative Studies
In addition to routine laboratory work, preoperative investigations include pulmonary function tests, because many patients may have predisposing factors such as a smoking history, Marfan syndrome with bullae formation, obstructive lesions related to the arch aneurysms (particularly with a congenital aberrant right subclavian artery lesions [see Fig. 68-2]), and right-sided aortic arches (see Fig. 68-3). Some patients are treated for a number of years for asthma because of expiratory wheezes caused by compression of the trachea or bronchi by aneurysms.47
Brain MRI or CT should be performed prior to surgery to detect any asymptomatic infarcts or brain lesions.62 Most patients undergo carotid ultrasound studies if a history of coronary artery disease, peripheral vascular disease, or left main coronary artery stenosis is present. All patients undergo echocardiography prior to surgery,5,15,32 and, if possible, good views of the ascending aorta and descending aorta are obtained by transesophageal echocardiography to check for atheromatous disease, as this will affect the method of cannulation for arterial inflow. All patients should also undergo cardiac catheterization to identify the presence of any coronary artery disease or valvular heart disease. If time allows, we obtain preoperative neurocognitive test results. Routine 24-hour Holter monitor examinations are included in the preoperative workup because many of these patients have cardiac arrhythmias, most likely related to valvular heart disease, chronic hypertension, coronary artery disease, or displacement of the left ventricle and the heart downward and to the left by the aneurysm. Patients with some syndromes, such as Marfan syndrome, exhibit more frequent prolonged QT intervals. Indeed, some aneurysms enlarge not only in diameter but also in length. Arrhythmias may also be related to abnormal vagal and sympathetic innervation stimulation.
CARDIOPULMONARY PERFUSION ASPECTS
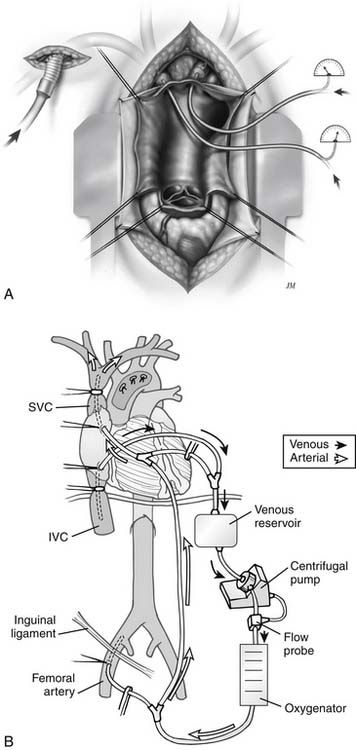
When the right subclavian artery is used, the technique is similar to that previously reported and shown in figure 68-8. In a prospective randomized study, it was thought this would be a useful means for antegrade brain perfusion.48,54 To do this, the innominate artery, in addition to the common carotid artery, was occluded with a balloon catheter. The balloon catheter in the common carotid artery allowed perfusion of the left side of the brain (see Fig. 68-8). This has proved to be a safe and effective technique.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree