Surgery of the Aorta
Acute Aortic Dissection
Acute aortic dissection has a sudden onset and is a true surgical emergency. It is usually initiated by a transverse tear in the intima or the intima and media. This disruptive injury gives rise to a hematoma within the media. The pulsatile force of ejection of the left ventricle causes a longitudinal separation of the aortic wall, mainly along and within the media. This dissection can progress both distally and proximally. Distal progression beyond the aortic arch can continue along the course of the descending thoracic and abdominal aorta to a variable extent and can involve its branches. Proximal extension of the dissecting hematoma may infiltrate the aortic root, distorting the aortic valve leaflets or compressing the ostia of the coronary arteries. This can produce aortic valve insufficiency and acute myocardial ischemia, respectively, both of which can cause death. In addition, acute dissection can cause rupture of the aorta into the pericardium. Therefore, the symptomatology of aortic dissection varies depending on its effect on the aortic valve, aortic wall, or aortic branches.
The underlying cause for the development of acute aortic dissection is associated with many factors. Of great significance is medial degeneration or cystic medial necrosis of the aortic wall. Marfan syndrome, an autosomal dominant disorder, is commonly associated with acute aortic dissection. However, annuloectasia can occur in patients without Marfan syndrome and result in acute aortic dissection. Of significant clinical importance is the association of hypertension, the presence of a bicuspid aortic valve, and coarctation of the aorta with acute aortic dissection.
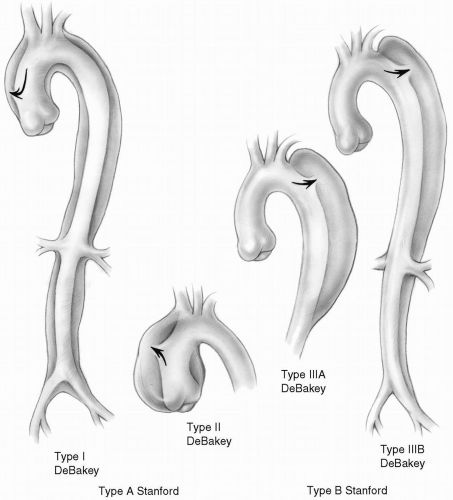
The current classification (Stanford) distinguishes two types of aortic dissection based on the involvement of the ascending aorta. Type A, or anterior, dissection commonly starts in the ascending aorta, usually 1 to 2 cm above the sinotubular junction, and may progress along the course of the aorta for a variable distance. Type B, or posterior, dissection typically starts in the descending aorta distal to the origin of the subclavian artery. The dissection can progress distally to a variable distance; less commonly it may extend proximally, thereby resulting in a type A dissection.
DeBakey classification is based on the anatomic location of the dissection. Therefore, type A Stanford classification conforms to DeBakey types I and II, whereas type B Stanford classification includes DeBakey types IIIA and IIIB (Fig. 8-1). From the practical point of view, the Stanford classification is simple and provides guidance as to the initial method of management (surgical versus medical) as well as surgical approaches (median sternotomy versus left lateral thoracotomy).
The immediate management of all acute aortic dissections is to reduce and maintain the patient’s systolic blood pressure at a level that still ensures satisfactory cerebral and renal perfusion. All patients suspected of acute aortic dissection should immediately undergo computed tomography with contrast. Acute type A aortic dissection is a surgical emergency because conservative therapy is not effective in most instances. On the other hand, patients with acute type B dissection are initially treated medically with antihypertensive therapy. There are occasions when the diagnosis of acute type A aortic dissection cannot be made with computed tomography. In these cases, transesophageal echocardiography should be performed to rule out the involvement of the ascending aorta in the dissection process. This can be accomplished in the intensive care unit, the emergency department, or the operating room.
Aortic Aneurysms
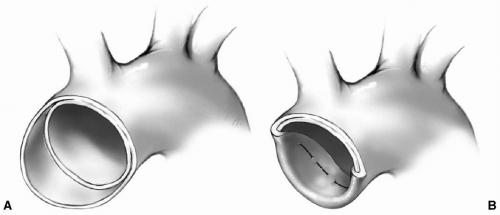
Aortic aneurysm is a localized enlargement and dilation of the arterial wall. It can affect any segment of the aorta. The prevalence of thoracic aortic aneurysms has tripled in the last 20 years. This increase in prevalence may be partly due to the increasingly aging population, better imaging, or an actual increase in the incidence. Thoracic aortic aneurysms are now estimated to affect 10 of every 100,000 elderly adults. The ascending aorta is most commonly affected (45%), followed by the descending aorta (35%). The aortic arch (10%) is involved either as an isolated lesion or as an extension of the ascending or, less commonly, descending thoracic aorta. Progressive enlargement of the aneurysm is an indication for resection and replacement with a tube graft because it will eventually rupture, culminating in the death of the patient.
The techniques for excision and graft replacement for aneurysms of the ascending and descending thoracic aorta are similar to those described for surgical management of types A and B aortic dissections. In addition, patients with a porcelain or severely atherosclerotic aorta requiring an aortic valve procedure may need replacement of the ascending aorta.
Replacement of the Ascending Aorta
The ascending aorta is approached by median sternotomy. Both groins should be in the operative field, and the arterial return is accomplished by cannulating either femoral or external iliac arteries. In patients with ascending aneurysms, direct aortic cannulation may be feasible. In many centers, the right axillary artery is used.
 The right femoral artery is less commonly involved in aortic dissection and therefore should be the site of choice for femoral cannulation.
The right femoral artery is less commonly involved in aortic dissection and therefore should be the site of choice for femoral cannulation.In patients with aortic dissection, the disease often extends distally, sometimes down to the femoral vessels; therefore, care must be exercised not to cannulate and perfuse through the false lumen of the femoral artery in a retrograde manner.
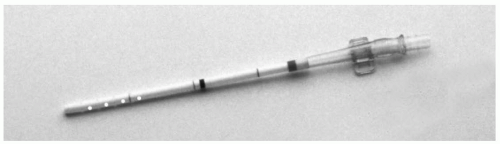
In elderly patients with severe atherosclerosis, the femoral and iliac arteries are markedly diseased and cannulation may be hazardous. Cannulas are available which can be introduced into these vessels percutaneously or under direct vision with a needle and wire technique (Fig. 8-2). The flow through a size 20 cannula is adequate for all but very large patients. Alternatively, the axillary artery may be used.
A dual-stage atriocaval cannula is usually used for venous drainage.
If the procedure is a reoperation, it is preferable to achieve cardiopulmonary bypass through a femoral
artery and femoral vein before opening the sternum (see Chapter 2).
artery and femoral vein before opening the sternum (see Chapter 2).
It is prudent to initiate cardiopulmonary bypass promptly by means of the femoral vessels before the administration of general anesthesia in patients with unstable hemodynamics to prevent circulatory collapse. This is especially important if pericardial tamponade is apparent or suspected.
Femoral vein cannulation can be achieved by introducing a long cannula with multiple side holes for excellent venous return. The important characteristic of this device is that it comes with a guidewire and contains a tapered, dilated sheath inside the cannula. The guidewire allows easy, comfortable, and safe passage of the cannula over the pelvic rim. The cannula has multiple side holes and may be advanced into the right atrium, providing superior drainage.
Venous cannulas that lack guidewires often hang up at the pelvic rim, resulting in inadequate venous return. If an attempt is made to push the cannula further into the inferior vena cava, perforation of the iliac vein with catastrophic consequences may ensue. It is usually easier to pass the cannula through the right femoral vein because of its straighter course compared with the left femoral vein.
After completion of a median sternotomy, an additional venous cannula is placed in the right atrium if required. A left ventricular vent through the right superior pulmonary vein (see Chapter 4) decompresses the heart and expedites the procedure. Venting is especially important if aortic valve insufficiency is present.
Retrograde Cerebral Perfusion
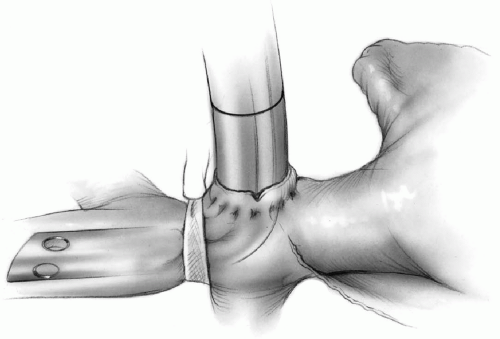
Whenever deep circulatory arrest is contemplated, the patient is generally cooled down to bladder temperature of 18°C to 24°C. Moderate hypothermia (bladder temperature of 26°C to 28°C) is safe if the anticipated period of circulatory arrest is less than 15 to 20 minutes. Ice is packed around the patient’s head. A tape is passed around the superior vena cava (see Chapter 2). A purse-string suture of 4-0 Prolene is applied to the adventitia of the superior vena cava at its junction with the pericardium. The adventitial tissue within the purse-string suture is cleaned off the superior vena cava, and an incision is made on the vein. The opening is enlarged with the tip of a clamp or scissors, and a long right-angled cannula is introduced into the superior vena cava and guided upward past the innominate vein (Fig. 8-3). This cannula is then connected to an arm of the cardioplegia delivery system or to the arterial line to perfuse cold blood into the superior vena cava whenever circulatory arrest is initiated. The tape around the superior vena cava is snugged down on the cannula to prevent perfusate from flowing back into the right atrium.
Tape is snugged down on the superior vena cava above the azygos vein to prevent runoff of cold blood into the azygos system (Fig. 8-3).
 Some thought has to be given to extending the concept of retrograde cerebral perfusion with cold blood to retrograde perfusion of the gastrointestinal tract and even the rest of the body. Consequently, at times, perfusion of cold blood through the azygos vein may be advantageous.
Some thought has to be given to extending the concept of retrograde cerebral perfusion with cold blood to retrograde perfusion of the gastrointestinal tract and even the rest of the body. Consequently, at times, perfusion of cold blood through the azygos vein may be advantageous.The central venous pressure should not exceed 30 to 40 mm Hg as measured by the side arm of the Swan-Ganz introducer in the internal jugular or subclavian vein. The perfusion flow should be approximately 400 to 800 mL per minute. It is not quite evident if the retrograde cerebral perfusion provides any nutritive support to the brain. However, it is clear that it does provide a uniform cooling of the brain. Its most important benefit is prevention of air or debris from flowing upward into the arch vessels, which would cause cerebral emboli. This can be appreciated when atherosclerotic debris is seen floating in the very dark desaturated blood flowing out of the arch vessels into the operative field.
At the end of circulatory arrest, retrograde cerebral perfusion is discontinued and the cannula is removed. The purse-string suture on the superior vena cava is tied down. If retrograde cerebral perfusion has been accomplished using an arm of the cardioplegia system, retrograde flow is continued for the first 1 to 2 minutes after resuming cardiopulmonary bypass to help prevent air embolism to the arch vessels.
Selective Antegrade Cerebral Perfusion
An alternative to retrograde cerebral perfusion is selective antegrade cerebral perfusion through the right axillary artery. In conjunction with innominate artery occlusion, this method can provide effective cerebral protection during circulatory arrest by allowing antegrade right carotid artery perfusion. Right axillary perfusion is also used for systemic perfusion during cardiopulmonary bypass.
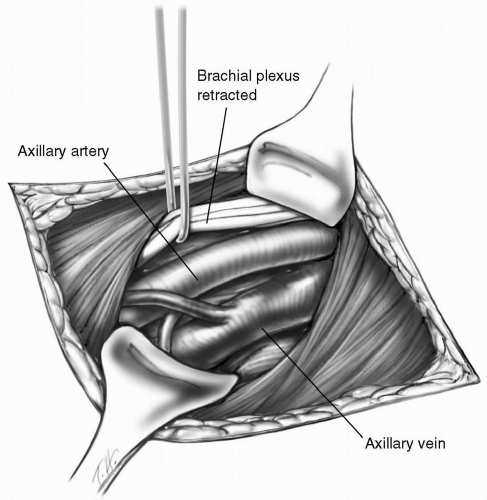
Before sternotomy, the right axillary artery is exposed through an 8 to 10 cm incision below and parallel to the lateral two-thirds of the clavicle. The pectoralis major muscle is divided in the direction of its fibers. The clavipectoral fascia is incised and the pectoralis minor muscle is retracted laterally. The axillary artery is located superior to the axillary vein. Using sharp dissection, the first part of the axillary artery is isolated. After administration of intravenous heparin, a small side-biting vascular clamp is applied to the artery. A longitudinal arteriotomy incision of approximately 1 cm is made and an 8-mm Hemashield Dacron tube graft (Medox Medical, Oakland, NJ) is sewn to the axillary artery in an end-to-side manner using 5-0 Prolene suture (Fig. 8-4). A 24-F arterial cannula is inserted into the graft and secured. Perfusion through a graft is safer than direct cannulation of the axillary artery and allows more accurate cerebral perfusion by monitoring the right radial artery pressure. During hypothermic circulatory arrest, axillary arterial blood flow is adjusted to maintain a radial artery pressure of 50 to 60 mm Hg.
Technique
On cardiopulmonary bypass with the heart decompressed, preliminary evaluation is made. The need for any concomitant additional procedures, such as coronary artery bypass grafting, must be noted. The conduct of the surgery should be choreographed precisely at this time.
When the nasopharyngeal temperature reaches 18°C to 24°C, the patient is placed in the Trendelenburg position. The heart-lung machine is halted, and retrograde cerebral perfusion or selective antegrade axillary perfusion is started. A transverse or longitudinal aortotomy is made on the anterior aspect of the aortic wall (Fig. 8-5). When dissection is present, the false lumen may be entered first. This necessitates opening of the true lumen.
Clamping of the Aorta
The aorta should be clamped only if there is a localized aneurysm of the ascending aorta with a generous normal distal segment. Only under this very precise condition should the aorta be cross-clamped. Deep circulatory arrest with retrograde cerebral perfusion is used when the ascending aortic aneurysm fades away into the arch or involves the arch as well, as in all patients with aortic dissection.
Application of a clamp to the aorta in the presence of acute aortic dissection further traumatizes the aortic
wall. In addition, it may pressurize the false lumen and result in progression of the dissection and possible obstruction of some aortic branches or even aortic rupture.
wall. In addition, it may pressurize the false lumen and result in progression of the dissection and possible obstruction of some aortic branches or even aortic rupture.
Blood clots are often evident within the aortic wall. In patients with aneurysms, the clots may be old and organized. They must be carefully removed along with atherosclerotic debris to prevent possible subsequent embolization.
Myocardial Protection
Cold blood cardioplegic solution may be administered antegrade into each coronary artery if deemed necessary. This is especially important if the dissection has involved one of the coronary ostia because the myocardium fed by this vessel may not have cooled sufficiently owing to obstructed flow. Retrograde infusion of cardioplegia into the coronary sinus should also be performed.
 If the cardioplegic line is used for the retrograde cerebral perfusion of cold blood, this will have to be delayed until the cardioplegic infusion is completed and the line purged of cardioplegic solution.
If the cardioplegic line is used for the retrograde cerebral perfusion of cold blood, this will have to be delayed until the cardioplegic infusion is completed and the line purged of cardioplegic solution.The entry site of the aortic dissection is identified. The dissection may have extended into the aortic arch and the aortic root involving a coronary ostium, most commonly that of the right coronary artery. The aorta is resected from just above the sinotubular ridge to the level of the innominate artery.
 The divided aortic wall may at times be left in situ to be reapproximated loosely over the tube graft at the completion of the procedure. This technique may provide added protection from possible mediastinal infection.
The divided aortic wall may at times be left in situ to be reapproximated loosely over the tube graft at the completion of the procedure. This technique may provide added protection from possible mediastinal infection.Typically, the lesser curvature of the aortic arch is resected to remove as much diseased aorta as possible. A 1-cm cuff of relatively normal aorta is dissected with as much adventitial tissue as possible left intact for the distal anastomosis.
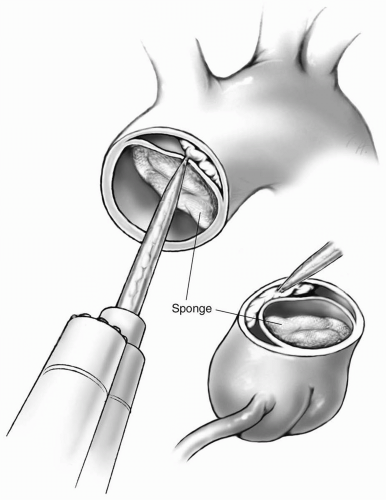
If the distal aortic wall is dissected, BioGlue Surgical Adhesive (CryoLife, Inc., Kennesaw, GA) is injected into the false lumen to bond and strengthen the aortic wall (Fig. 8-6). A sponge is placed in the true lumen to prevent spillage.
 The sponge within the lumen of the aorta is gently pressed against the aortic wall in close proximity to the coronary ostia to prevent the glue material from occluding the coronary arteries.
The sponge within the lumen of the aorta is gently pressed against the aortic wall in close proximity to the coronary ostia to prevent the glue material from occluding the coronary arteries.Glue material is not introduced within the dissected distal wall of the aorta if there appears to be reentry sites within the aortic arch. The possibility of glue material becoming detached and embolized through the distal reentry site is a grave complication of this procedure.
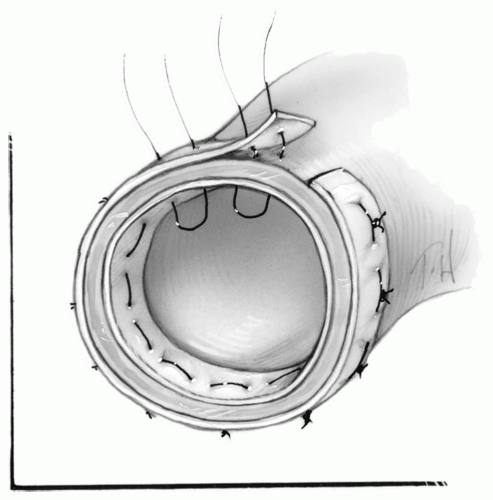
Further reinforcement can be obtained with Teflon felt strips attached to both the inside and/or outside of the aortic wall first with 6 to 10 interrupted mattress sutures or a continuous mattress suture of 3-0 Prolene (Fig. 8-7). Teflon felt strips may not be required if the integrity of the aortic wall appears to be satisfactory with the glue. Alternatively, the outer adventitial layer of the dissected aorta can be cut longer than the inner intimal layer. This layer is then folded into the true lumen and sewn in place with interrupted mattress sutures (Fig. 8-8).
An appropriately sized Hemashield tube graft is cut and tailored obliquely to be attached to the undersurface of the arch or cut straight to be attached to the aorta at the level of the innominate artery. The tube graft is then anastomosed to the reinforced aortic cuff with a continuous 3-0 Prolene suture.
It is important for the assistant surgeon to follow the suture meticulously to provide appropriate tension on the suture line. Otherwise, multiple reinforcing interrupted sutures may be required to ensure a watertight anastomosis.
With the patient in the Trendelenburg position, the perfusion of retrograde cerebral blood is allowed to accumulate and fill the aortic arch. All air and debris are allowed to flow out through the graft. At this time, another arterial cannula is introduced through the tube graft, and the perfusionist is asked to initiate arterial perfusion through this cannula in an antegrade manner with extremely low flow. A clamp is now applied to the tube graft well away from the anastomosis and proximal to the cannula, and the retrograde cerebral perfusion is gradually discontinued and venous drainage is reinstituted (Fig. 8-9). Normal perfusion flow and pressure are gradually restored, and the patient is rewarmed. The posterior distal suture line is now examined, and additional stitches are placed for control of hemostasis if required.
 In patients with aortic aneurysm, the femoral arterial cannula may be used to reinstate cardiopulmonary bypass. This retrograde arterial perfusion is gradually increased to normal flow, and rewarming is started. Antegrade perfusion is not essential. However, antegrade perfusion with a separate cannula through the tube graft allows earlier removal of the femoral arterial cannula and repair of the femoral artery while the remainder of the surgery is being completed.
In patients with aortic aneurysm, the femoral arterial cannula may be used to reinstate cardiopulmonary bypass. This retrograde arterial perfusion is gradually increased to normal flow, and rewarming is started. Antegrade perfusion is not essential. However, antegrade perfusion with a separate cannula through the tube graft allows earlier removal of the femoral arterial cannula and repair of the femoral artery while the remainder of the surgery is being completed.In patients with aortic dissection, blood gains access through the entry site into the aortic wall. This dissection may result in a reentry site by tearing the intima distally along the course of the aorta. When cardiopulmonary bypass is reinitiated, the retrograde flow may enter the false lumen through this distal intimal tear and reenter the lumen at the entry site. However, when the aorta has been repaired and the entry site is excluded by tube graft interposition, the retrograde flow of blood cannot escape and may cause further dissection of the aorta. Therefore, it is important to establish antegrade flow when restarting cardiopulmonary bypass.
 If right axillary artery cannulation has been used, the tube graft can be filled by removing the clamp on the innominate artery. The graft is then cross-clamped, and full flow is resumed.
If right axillary artery cannulation has been used, the tube graft can be filled by removing the clamp on the innominate artery. The graft is then cross-clamped, and full flow is resumed. After cardiopulmonary bypass is reestablished, additional doses of blood cardioplegic solution are administered by the retrograde technique and antegrade into the coronary ostia at 10- to 20-minute intervals (see Chapters 3 and 5).
After cardiopulmonary bypass is reestablished, additional doses of blood cardioplegic solution are administered by the retrograde technique and antegrade into the coronary ostia at 10- to 20-minute intervals (see Chapters 3 and 5).When the aorta is otherwise normal and there is no aortic valve insufficiency, the proximal aorta that has




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree