Chapter 14 Surgery in the Geriatric Patient
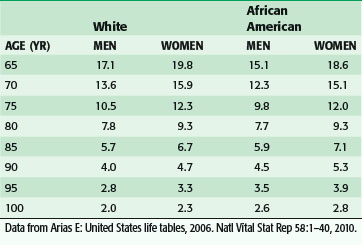
Over the past several generations, life expectancy in the United States has increased significantly. This is primarily the result of the implementation of public health and medical interventions such as improved sanitation, vaccinations, nutrition and lifestyle modifications, and antibiotics. From 1900 to the present, life expectancy at birth has increased almost 30 years (49.2 to 77.8 years), and the average 65-year-woman today can expect to live almost twice as long as her counterpart in 1900, or almost 20 years more (Table 14-1).1
With this increase in life expectancy comes an increase in the number of people living into old age with diseases and chronic conditions that would have caused death in earlier years. At present, more than 75% of those older than 65 years have at least one chronic condition and 20% of the Medicare population have 5 or more.2 Many of these diseases and chronic conditions, such as cancer, degenerative joint disease, coronary artery disease, and visual impairment, have a surgical option as part of the treatment algorithm.
Over the next few decades, as the 78 million people in the Baby Boomer generation (born from 1946 to 1964) begin to reach age 65, there will be a rapid aging of the U.S. population (Fig. 14-1).3 It is expected that by 2030, one in five people will be older than 65 years and, by 2050, almost 20 million people will be older than 85 years. Currently, Social Security, Medicare, and Medicaid benefits to older adults account for more than one third of U.S. spending and have the potential to consume the entire federal budget in the near future. Over the next decade, Medicare costs alone are expected to increase by 7%/year (Fig. 14-2).4 The demand for health care services is likely to overwhelm the system if new ways to increase supply and delivery are not developed.
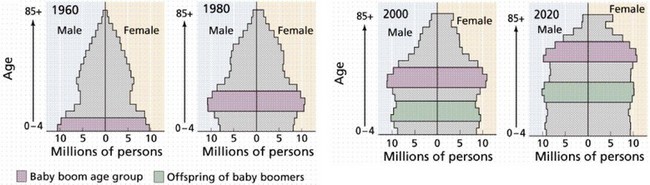
FIGURE 14-1 Aging of the Baby Boom generation and offspring.
(From Purves WK, Orians GH, Heller HC: Life: The science of biology, ed 4, New York, 1994, WH Freeman.)
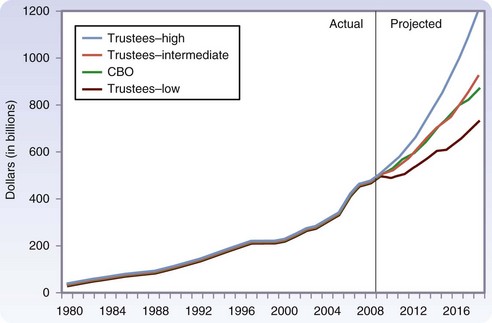
FIGURE 14-2 Expected growth in Medicare spending over the next decade.
(From Medicare Payment Advisory Commission: A data book: Healthcare spending and the Medicare program, MedPAC, 2009, Washington DC.)
In April 2008, in response to the impending crisis in providing health services to older citizens, the Institute of Medicine (IOM) issued a report, in which the IOM “charged the Committee on the Future Health Care Workforce for Older Americans with determining the health care needs of Americans over 65 years of age and analyzing the forces that shape the health care workforce for these individuals.”2 The committee determined that to meet these needs, a three-pronged approach was necessary:
Aging and Surgery
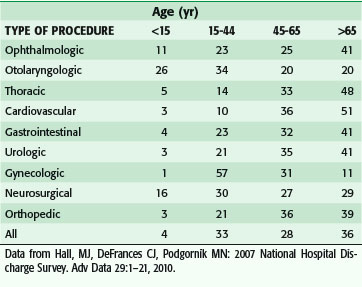
As the number of persons reaching old age continues to grow, there will be a concomitant need to provide surgical care to an increasing number of older patients. Over the past 2 decades alone, the percentage of surgeries in which the patient was older than 65 increased from 19% to 35% of all operations. When obstetric procedures are excluded, this portion rises to 43%. The proportion of the surgical workload across age groups in the specialties in non–federally funded hospitals is shown in Table 14-2.5
Table 14-2 Distribution of Procedure Types (by Age Group) Performed in Non–Federally Funded Hospitals (%)
The pattern of surgical management of malignant disease in older adults is an example of the changing views on surgery in this age group. Data from the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) program have indicated a decrease in the gap between the percentage of younger and older patients treated surgically for certain cancers. For early-stage breast, colon, and rectal cancers, in which the chance of surgical cure is high, the percentage of older patients receiving surgical treatment has approached that of younger patients. For localized gastric, pancreatic, lung, and liver cancers, operative percentages still decline sharply with age (Fig. 14-3).6 At present, it is still unclear whether this decline is the result of appropriate decision making based on the overall health of the patient and patient treatment preference, or whether it is a reflection of vestigial prejudice and age bias resulting in patients not being referred for cancer surgery.
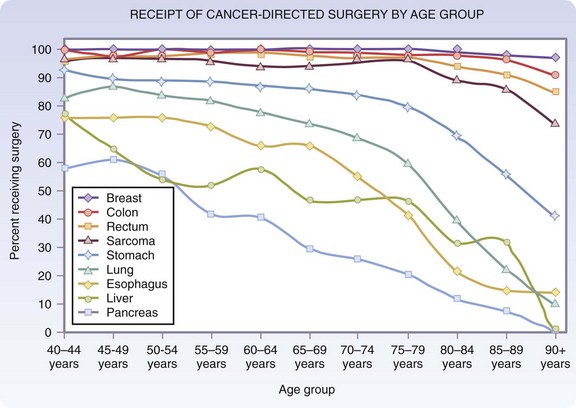
FIGURE 14-3 Age distribution of patients receiving cancer-directed surgery for local stage disease.
(Adapted from O’Connell JB, Maggard MA, Ko CY: Cancer-directed surgery for localized disease: Decreased use in the elderly. Ann Surg Oncol 11:962–969, 2004.)
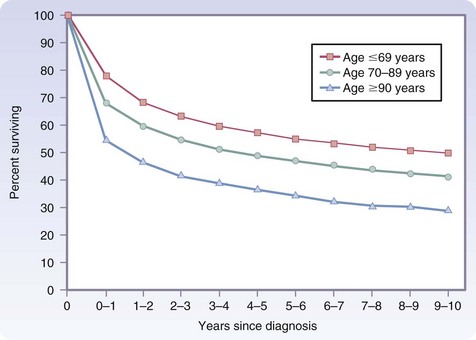
However, additional data from the SEER database indicate that even in the oldest patients, those 90 years and older, cancer treatment is worthwhile. After the first year from diagnosis, relative survival, defined as the ratio of observed survival over a specific period to expected survival, is identical for older and younger cancer patients for up to 10 years (Fig. 14-4).7 Most of the difference seen in the first year occurs in the first few months, and the only factor that positively influences first-year survival is whether the patient underwent surgical treatment of the cancer or other major surgery. This finding may be the result of selection bias, inasmuch as only the healthiest 90-year-olds may have been offered surgery. However, this serves to emphasize that age alone should not be the sole reason to deny surgical treatment of cancer.
There is no doubt that increasing age appears to have a negative effect on the outcome of surgery. However, most studies have indicated that chronologic age alone has little effect on outcome. Rather, it is the age-related decline in physiologic reserves and increase in comorbidity that is responsible for this observation. Even a compromised older patient can tolerate a surgical experience well if the procedure is carefully conducted and the postoperative course is uncomplicated. However, if even one complication occurs, mortality increases significantly. In a study of more than 26,000 patients older than 80 years undergoing major noncardiac surgery in Veterans Affairs Hospitals, mortality rose from 3.7% in patients with no complications to 26.1% in patients in whom one or more complication occurred.8
Setting Goals for Treatment
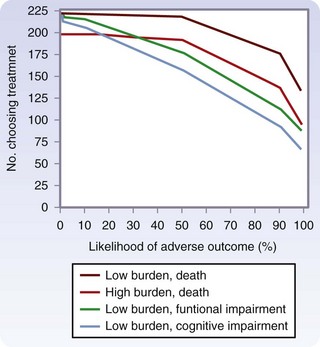
In a study of older patients with limited life expectancy because of serious chronic disease, Fried and colleagues9 examined the impact of treatment burden (low, minor interventions, such as IV antibiotics; high, major interventions, such as surgery) and expected outcome (desirable versus undesirable) on patient preferences for treatment. Results indicated that more than 70% of older patients would not want even a low burden treatment if severe functional impairment or cognitive impairment was the expected outcome. The concern for functional and cognitive impairment was more dramatic than the concern for death (Fig. 14-5).
In another study of preferences for permanent nursing home placement in seriously ill hospitalized patients, 56% of patients were very unwilling or would rather die than live permanently in a nursing home. Correlation between the patient’s wishes and both the surrogate’s and physician’s opinion of the patient’s wishes was poor.10
Physiologic Decline
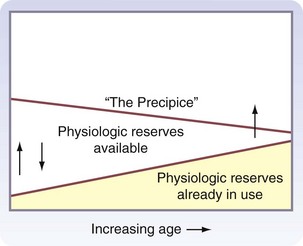
With aging, there is a decline in physiologic function in all organ systems, but the magnitude of this decline is variable among organs and individuals. In the resting state, this decline usually has minimal functional consequence, although physiologic reserves may be used just to maintain homeostasis. However, when physiologic reserves are required to meet the additional challenges of surgery or acute illness, overall performance may deteriorate. This progressive age-related decline in organ system homeostatic reserves, termed homeostenosis, was first described by the physiologist Walter Cannon in the 1940s. Figure 14-6 is a graphic representation of the present concepts of homeostenosis.11 With older age, there is increased use of physiologic reserves just to maintain normal homeostasis. Therefore, when stressed, fewer reserves are available to meet the challenge, and overall function may be pushed over the precipice of organ failure or death.
Cardiovascular System
Cardiovascular disease is the leading cause of death in the United States in men and women. Of these deaths, 83% occur in persons older than 65 years (Box 14-1). The prevalence of heart failure approaches 10 in 1000 persons in this age group. Congestive heart failure is a risk factor for several postoperative complications, including surgical site infections. Cardiac events are common in the postoperative period in older patients and are attributable to disease and to changes in the structure and function of the heart that accompany aging.
Box 14-1 Major Cardiovascular Changes With Age
Fibrosis of conducting pathways with increased arrhythmias
Decrease ventricular and arterial compliance (increased afterload)
Decreased β-adrenergic responsiveness
Increased dependence on preload (including atrial kick)
Increased diastolic dysfunction
Diastolic function, however, which depends on relaxation rather than contraction, is affected by aging.12 Diastolic dysfunction is responsible for up to 50% of cases of heart failure in patients older than 80 years. Myocardial relaxation is more energy-dependent and therefore requires more oxygen than contraction. With aging, there is a progressive decrease in the partial pressure of oxygen. Consequently, even mild hypoxemia can result in prolonged relaxation, higher diastolic pressure, and pulmonary congestion. Because early diastolic filling is impaired, maintenance of preload becomes even more reliant on atrial kick. Loss of the atrial contribution to preload can result in further impairment of cardiac function.
Respiratory System
Chronic lower respiratory disease is the fourth leading cause of death after heart disease, cancer, and stroke. Respiratory problems are the most common postoperative complications in older patients (Box 14-2). Both disease- and age-related changes in lung structure and function contribute to this vulnerability.13
Box 14-2 Major Respiratory Changes With Age
Decrease chest wall compliance
Decline in maximum inspiratory and expiratory force
Decrease in lung elasticity (small airway collapse)
Ventilation-perfusion mismatch
Decrease in PaO2, no change in PaCO2
Decline in ventilator responses to hypoxemia and hypercapnia
Decline in normal airway protective mechanisms (increased risk for aspiration)
Renal System
Approximately 25% of all Americans 70 years and older have moderately or severely decreased kidney function (Box 14-3). Between the ages of 25 and 85, there is a progressive decrease in the renal cortex. Over time, approximately 40% of the nephrons become sclerotic. The remaining functional units hypertrophy in a compensatory manner. Sclerosis of the glomeruli is accompanied by atrophy of the afferent and efferent arterioles and by a decrease in renal tubular cell number. Renal blood flow also falls by approximately 50%. Functionally, there is a decline in the glomerular filtration rate (GFR) of approximately 45% by age 80 years.
Box 14-3 Major Renal Changes With Age
Decrease in the number of functional nephrons
Decrease in the number of tubular cells
Decline in creatinine clearance despite normal serum creatinine level
Decline in tubular function (loss of concentrating ability)
Increase susceptibility to dehydration
Decrease clearance of certain drugs
Renal tubular function also declines with advancing age. The ability to conserve sodium and excrete hydrogen ion decreases, resulting in a diminished capacity to regulate fluid and acid-base balance. Dehydration becomes a particular problem because losses of sodium and water from nonrenal causes are not compensated for by the usual mechanisms. The inability to retain sodium is believed to be caused by a decline in the activity of the renin-angiotensin system. The increasing inability to concentrate the urine is related to a decline in end-organ responsiveness to antidiuretic hormone. The marked decline in the subjective feeling of thirst is also well documented but not well understood. Alterations of osmoreceptor function in the hypothalamus may be responsible for the failure to recognize thirst in spite of significant elevations in serum osmolality.14
Because of the decline in renal function with aging, it is often important to measure GFR in older patients as part of preoperative risk assessment and in hospital to provide accurate medication dosing. In older hospital patients, direct measurement of creatinine clearance (CrCl) is difficult because incontinence and cognitive impairment make 24-hour urine collection unreliable. Serum creatinine level measurement may be an unreliable indicator of renal function status because this value may remain unchanged as a result of a concomitant decrease in lean body mass and thus a decrease in creatinine production. A serum creatinine level of 1.0 mg/dL may represent a CrCl of over 100 mL/min in a 30-year old, but less than 60 mL/min in an 85-year old.15
To overcome these problems, formulas have been developed to estimate CrCl from plasma creatinine and patient characteristics. The most commonly used formulas are the Cockcroft-Gault equation and the Modification of Diet in Renal Disease (MDRD) equation (Fig. 14-7). In a large study of older hospitalized patients, the Cockcroft-Gault equation has been shown to correlate more closely with directly measured CrCl.16
Hepatobiliary System
Overall, hepatic function is well preserved with aging. However, there is as much as a fourfold increase in liver disease–related mortality in persons between the ages of 45 and 85 years.17 Morphologic changes include a decrease in the number of hepatocytes and a reduction in overall weight, size, and volume. There is, however, a compensatory increase in cell size and proliferation of bile ducts. Functionally, hepatic blood flow decreases by approximately 0.3% to 1.5%/year to 40% to 45% of earlier values after 65 years of age.
The synthetic capacity of the liver, as measured by standard tests of liver function, remains unchanged (Box 14-4). However, the metabolism of and sensitivity to certain types of drugs is altered. Drugs requiring microsomal oxidation (phase I reactions) before conjugation (phase II reactions) may be metabolized more slowly, whereas those requiring only conjugation may be cleared at a normal rate. Drugs that act directly on hepatocytes, such as warfarin (Coumadin), may produce the desired therapeutic effects at lower doses in older adults because of an increased sensitivity of cells to these agents. Some recent evidence has also suggested that aging may be associated with a decline in the ability of the liver to protect against the effects of oxidative stress.
Box 14-4 Major Hepatobiliary Changes With Age
Decrease in the number of hepatocytes
Synthetic capacity remains unchanged
Increased sensitivity to and decreased clearance of certain drugs
Increased incidence of gallstones and gallstones related diseases
Immune Function
Immune competence, like other physiologic parameters, declines with advancing age (Box 14-5). This immunosenescence is characterized by enhanced susceptibility to infections, an increase in autoantibodies and monoclonal immunoglobulins, and an increase in tumorigenesis. In addition, like other physiologic systems, this decline may not be apparent in the unchallenged state. For example, there is no decline in neutrophil count with age, but the ability of the bone marrow to increase neutrophil production in response to infection may be impaired. Older patients with major infections frequently have normal white blood cell (WBC) counts, but the differential count will show a profound shift to the left, with a large proportion of immature forms.
Box 14-5 Major Changes in Immune Function With Age
Decrease production and differentiation of naïve T cells
Decrease in T cell mitogenic activity
Increase in inflammatory cytokines
With aging, there is a decline in the hematopoietic stem cell pool in the bone marrow that leads to decreased production of naïve T cells from the thymus and of B cells from the bone marrow. Moreover, involution of the thymus gland, with a decline in thymic hormone levels, further impairs the production and differentiation of naïve T cells and leads to an increased proportion of memory T cells. This change in the population of T cells leaves older adult hosts less able to respond to new antigens. Furthermore, recent data have suggested that chronic infection with viruses such as cytomegalovirus produces nonfunctional T cell clonal expansions that may limit the space available for proliferating T cells.18
Glucose Homeostasis
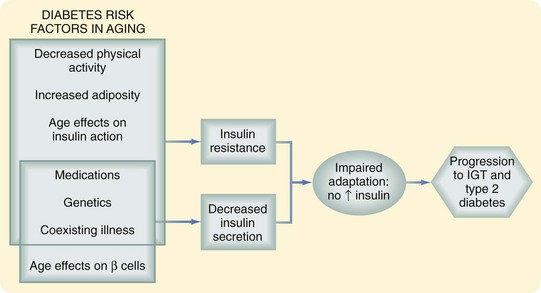
Data from the National Health and Nutrition Examination Survey have shown a clear increase in the prevalence of disorders of glucose homeostasis with age; more than 20% of persons older than 60 years have type 2 diabetes. An additional 20% have glucose intolerance characterized by normal fasting glucose and a postchallenge glucose level higher than 140 mg/dL but less than 200 mg/dL. This glucose intolerance may be the result of a decrease in insulin secretion, increase in insulin resistance, or both (Fig. 14-8).19
There is now general consensus that beta cell function declines with age. This change is manifested by failure of the beta cell to adapt to the hyperglycemic milieu with an appropriate increase in insulin response. The question of insulin resistance is more controversial. Although insulin action has been shown to decrease in older adults, this change is thought to be more a function of changing body composition, with increased adipose tissue and decreased lean body mass, rather than age per se. Others believe that there is an increase in insulin resistance directly attributable to aging, as manifested by a decrease in insulin-mediated glucose uptake in muscle that is normally regulated by the glucose transporter GLUT-4. There is also an increase in intracellular lipid accumulation, which interferes with normal insulin signaling. These changes may be associated with the decline in mitochondrial function that also accompanies aging.19
Preoperative Assessment
The goals of preoperative assessment of an older patient are to define the extent of physiologic decline, characterize and optimize comorbid diseases, and determine how the stress of the surgical treatment will affect the patient’s postoperative function and overall quality of life. Extensive testing for disease in every organ system is neither cost-effective, practical, nor necessary for most patients. A thorough history and physical examination will provide information to direct further workup, if necessary. It is important, however, to adjust the history and physical examination to look carefully for the risk factors, signs, and symptoms of the more common comorbid conditions. The addition of simple tools for the assessment of functional, cognitive, nutritional status and overall frailty will significantly enhance understanding of the individual patient’s true operative risk (Box 14-6). When initial evaluation identifies specific disease or risk factors for disease, further workup may be indicated.
Comorbidity
Of all comorbid conditions, cardiovascular disease is the most prevalent, and cardiovascular events are a leading cause of severe perioperative complications and death. Therefore, the main thrust of preoperative evaluation in most patients, regardless of age, has focused on identifying patients at risk for cardiac complications. The American College of Cardiology (ACC) and American Heart Association (AHA) Task Force on Practice Guidelines first published an in-depth set of guidelines for preoperative cardiac evaluation in 1996, with updates in 2002 and 2007.20 These guidelines provide a stepwise Bayesian strategy for determining which patients will need further testing to clarify risk or further treatment to minimize risk. Stratification is based on factors related to the patient and type of surgery. For older patients with known cardiac disease, rigorous workup may be necessary. For most patients, assessment of exercise tolerance and functional capacity is an accurate method of predicting the adequacy of cardiac and pulmonary reserves (see later).
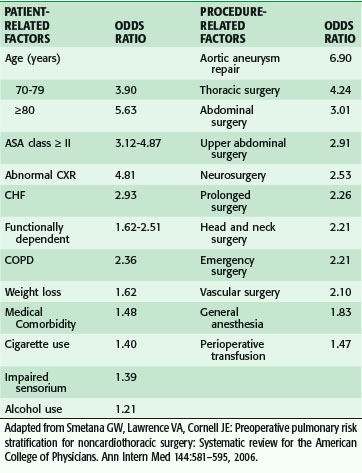
Although the main focus of preoperative evaluation has been cardiac status, pulmonary complications in older patients are at least as common as cardiac complication, if not more so. Risk factors for pulmonary complications are not as well studied as those for cardiac complications, although many of the same issues apply to both. Poor exercise capacity and poor general health predict pulmonary and cardiac complications. In a systematic review of the literature for risk factor for pulmonary complications after noncardiac surgery (not limited to older adults) both patient and procedural factors were identified (Table 14-3).21 Age older than 80 years was associated with the highest odds ratio (OR) of a pulmonary complication, even after adjusting for comorbidity. Indicators of impaired function, nutrition, and cognition, among others, were also important. Aortic aneurysm and thoracic and abdominal operations were the strongest procedure-related factors, but others, such as abdominal surgery, prolonged surgery, and emergency surgery, were also important.
In a recent review of strategies to reduce postoperative pulmonary complications, only lung expansion interventions, such as incentive spirometry, were shown by good evidence to have an effect.22 The selective use of nasogastric decompression (rather than routine use) and short-acting (as opposed to long-acting) intraoperative neuromuscular blockade was supported by fair evidence. Evidence supporting smoking cessation, epidural anesthesia and analgesia, laparoscopic versus open approaches, and nutritional supplementation was insufficient or conflicting.
Function
American Society of Anesthesiologists Classification
For decades, the physical status classification of the American Society of Anesthesiologists (ASA) has been used successfully to stratify operative risk. This simple classification ranks patients according to the functional limitations imposed by coexisting disease. When curves for mortality versus ASA class are examined with regard to age, there is little difference between younger and older patients, which indicates that mortality is a function of coexisting disease rather than chronologic age. ASA classification has been shown to predict postoperative mortality accurately, even in patients older than 80 years. In a large multicenter Department of Veterans Affairs study (National Surgical Quality Improvement Program [NSQIP]), surgical patients were assessed prospectively for operative risk and risk-adjusted models were then created to allow comparison of the quality of surgical care among different institutions. Of the 68 variables studied, the ASA functional classification was the factor most predictive of postoperative morbidity and the second most predictive of mortality.23
Activities of Daily Living
The ability to perform ADLs (e.g., feeding, continence, transferring, toileting, dressing, bathing) and instrumental ADLs (IADLs; e.g., telephone use, transportation, meal preparation, shopping, housework, medication management, managing finances) have also been shown to correlate with postoperative mortality and morbidity.24 Inactivity, defined as the inability to leave the home on one’s own at least twice per week, has been associated with a higher incidence of all major surgical complications. Postoperative mortality in severely limited patients has been reported to be almost 10 times higher than mortality in active patients. In another study of functional recovery after major elective open abdominal operations, better recovery and shorter time to recovery of ADLs and IADLs were almost always predicted by a better preoperative physical performance status, as measured by three simple tests of strength and mobility.25
Exercise Tolerance
Of all the methods of assessing overall functional capacity, exercise tolerance is the most sensitive predictor of postoperative cardiac and pulmonary complications in older adults. In an older but frequently quoted study comparing exercise tolerance and other assessment techniques, Gerson and associates demonstrated that an inability to raise the heart rate to 99 beats/min while performing 2 minutes of supine bicycle exercise was the most sensitive predictor of postoperative cardiac and pulmonary complications and death.26
Formal exercise testing, however, is not necessary in every older patient. The metabolic requirements for many routine activities have already been determined and are quantitated as metabolic equivalents (MET). One MET, defined as 3.5 mL/kg/min, represents the basal oxygen consumption of a 70-kg, 40-year-old man at rest. Estimated energy requirements for various activities are shown in Figure 14-9. An inability to function above 4 METs has been associated with increased perioperative cardiac events and long-term risk. By asking appropriate questions about the level of activity, functional capacity can be accurately determined without the need for additional testing.
Cognition
There are several methods for evaluating baseline cognitive function. The Folstein Mini- Mental State Examination (MMSE) has traditionally been used because of its ease of administration and reliability. It has been suggested that the Mini-Cog test detects clinically significant cognitive impairment as well as, if not better than, the MMSE in multiethnic older individuals.27 It is easier to administer to non–English-speaking patients and is less biased by low education and literacy levels. The Mini-Cog test combines a three-item word learning and recall task (0 to 3 points; each correctly recalled word, 1 point), with a simple clock-drawing task (abnormal clock, 0 points; normal clock, 2 points) used as a distraction before word recall. Total possible Mini-Cog scores range from 0 to 5 points, with 0 to 2 suggesting high and 3 to 5 suggesting a low likelihood of cognitive impairment.
Nutritional Status
The impact of poor nutrition as a risk factor for perioperative mortality and morbidity such as pneumonia and poor wound healing has long been appreciated. A variety of psychosocial issues and comorbid conditions common to older adults place this population at high risk for nutritional deficits. Malnutrition is estimated to occur in approximately 0% to 15% of community-dwelling older persons, 35% to 65% of older patients in acute care hospitals, and 25% to 60% of institutionalized older adults. Factors that lead to inadequate intake and uptake of nutrients in this population include the ability to obtain food (e.g., financial constraints, availability of food, limited mobility), desire to eat food (e.g., living situation, mental status, chronic illness), ability to eat and absorb food (e.g., poor dentition, chronic gastrointestinal disorders such as GERD or diarrhea), and medications that interfere with appetite or nutrient metabolism (Box 14-7).