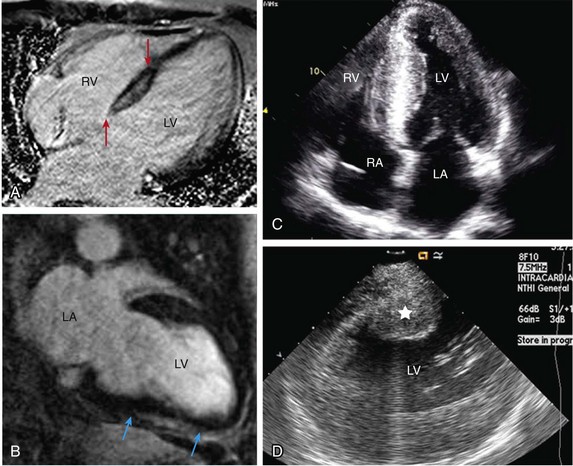
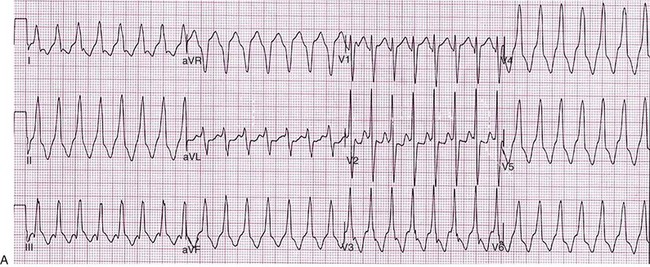
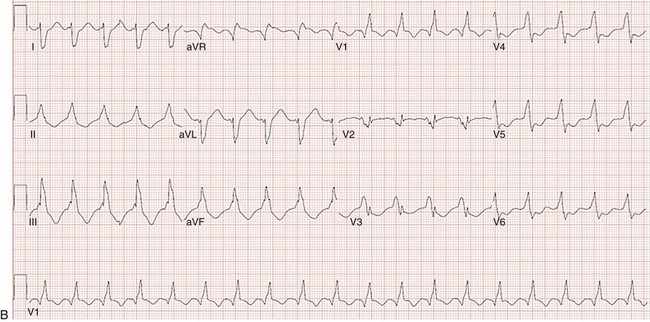
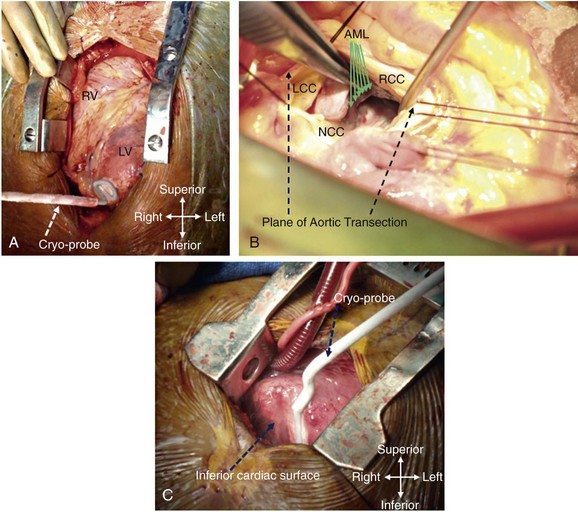
129 In the past, open-heart surgery was not uncommonly used to treat refractory ventricular arrhythmias.1 The predominant population subjected to this management strategy was comprised of patients with healed myocardial infarcts who experienced sustained ventricular tachycardia (VT). The original technique used resection of the dyskinetic/akinetic scarred myocardial tissue (aneurysmectomy) with only modest success (~40%) for long-term arrhythmia control.2 Subsequent mapping studies revealed that critical components of the VT reentrant circuit used the border zone between the dense scar and healthy myocardium. This resulted in the development of subendocardial resection (removing tissue up to a depth of 2-4 mm in the area surrounding the dense aneurysm).3 By using this approach with or without aneurysmectomy, the overall success rate for long-term arrhythmia control improved to 90%.4 Subsequent innovations in mapping tools (multielectrode epicardial shock and endocardial basket) allowed operators to better characterize VT circuits for more effective surgical ablation.5 Nevertheless, a major limitation of the technique remained its highly invasive nature (median sternotomy with cardiopulmonary bypass and ventriculotomy).6 Furthermore, because it was typically performed on sick patients with impaired cardiac function (who experienced recurrent ventricular arrhythmias refractory to multiple antiarrhythmic drugs), there was high periprocedure mortality rate.1,4,5,7 For these reasons, the technique was never widely adopted and it remained confined to a few centers/operators specializing in VT surgery. With the invention of implantable cardioverter defibrillators (ICDs), there was a shift away from surgical VT ablation. Furthermore, because ICDs could be implanted outside the operating room (OR), this technique was widely adopted. At the same time that ICD platforms were becoming smaller and more sophisticated, there were major advances in percutaneous catheter mapping and ablation techniques. The latter allowed electrophysiologists to replicate the surgical VT experience by using a significantly less invasive approach.8 These developments have resulted in the decline of surgical VT ablation, which nowadays is used only rarely. Nevertheless, in a select group of patients, surgical ablation is still an important option for treating refractory ventricular arrhythmias. Currently, surgical VT ablation is generally the treatment of last resort reserved primarily for patients who have failed a combination of antiarrhythmic drugs and percutaneous catheter ablation attempts.8,9 The experience at the Hospital of The University of Pennsylvania is consistent with this. Over a 3-year period (2007 to 2009), 527 patients underwent 644 VT ablation procedures; structural heart disease was present in 295 (56%). Of these 295, 144 patients (49%) had heart disease categorized as nonischemic based on the lack of a prior infarct and absence of coronary disease. In 8 of these patients (1.5%; 7 men), all with a nonischemic substrate (median left ventricular ejection fraction of 35%), the arrhythmia remained refractory to medications and endocardial/epicardial percutaneous radiofrequency (RF) ablation attempts. These patients eventually underwent surgical VT ablation for arrhythmia control. Six had dilated cardiomyopathy and 2 had longstanding hypertrophic cardiomyopathy.10 In the latter group, arrhythmia was found to originate from the midmyocardium (identified by magnetic resonance imaging [MRI]) of the basal left ventricle (LV), which was markedly thickened (>20 mm). This was impossible to target effectively by percutaneous catheter ablation from the endocardium or epicardium. Consistent with those observations, case reports from other investigators also suggest that ventricular arrhythmias arising from the basal interventricular septum (IVS) are challenging to ablate percutaneously.11 Thus, in patients with VT features manifesting on an electrocardiogram (ECG) that suggest origin from the IVS region, attempts should be made a priori to identify septal scar using MRI, positron emission tomography scan, and transthoracic and/or intracardiac echocardiography, among others (Figure 129-1). Typical ECG features suggesting VT origin from the IVS region include (1) left bundle branch block morphology with superior or inferior axis and early precordial transition (before lead V3) or (2) right bundle branch block morphology with inferior or superior axis and an unusual precordial transition pattern with lead V2 manifesting predominantly negative complexes (rS or QS) compared with leads V1 and V2 (Figure 129-2).12,13 In these cases, especially if a scar is identified in the IVS, it is prudent to make the patient aware of the possibility that percutaneous catheter ablation may not be successful. Similarly, in patients with hypertrophied LV who have ventricular arrhythmias in the setting of midmyocardial scar, consideration should be given to a surgical option if an attempt at percutaneous catheter ablation fails.10 In addition to this, surgical ablation of ventricular arrhythmias should be considered in patients who are undergoing open-heart surgery for other cardiac conditions, such as valve surgery or coronary artery bypass grafting, and have manifested recurrent VT despite antiarrhythmic drug (AAD) therapy and/or percutaneous catheter ablation attempts. Figure 129-1 Septal scar identified by MRI and echocardiographic imaging . A, Proximal interventricular scar (red arrows). B, Scar in the distal interventricular distribution extending to the apex (blue arrows) identified by MRI. C, Apical 4-chamber transthoracic echocardiographic view showing thickened septum with marked echogenicity representing infiltrative disease process. D, Thickened proximal interventricular septum (star) identified using intracardiac echocardiography in a patient with hypertrophic cardiomyopathy. In all these patients, refractory ventricular arrhythmias originated from the septal substrate, which was targeted during surgical ablation. LA, left atrium; RA, right atrium; RV, right ventricle; LV, left ventricle. Figure 129-2 A, 12-lead ECG of VT manifesting a left bundle branch block morphology and inferior axis with early transition (≤V3). B, 12-lead ECG of VT manifesting a right bundle branch block morphology, inferior axis, and unusual transition pattern (predominantly positive forces in leads V1, V3-V6 but negative forces in lead V2). These features are characteristic of VT originating from the interventricular septal region. Adequate access to all cardiac surfaces is critical to the success of surgical VT ablation. Median sternotomy typically provides the best visualization of the entire heart.1,4,10 The epicardial aspect of the right ventricle (RV), superior IVS, and anterior LV wall are well-visualized through median sternotomy without additional cardiac manipulation (Figure 129-3). However, to adequately inspect the posterolateral LV wall, the heart has to be physically lifted. Although this can cause a drop in cardiac output and blood pressure, proper pericardial and cardiac manipulation as conducted during off-pump coronary artery bypass grafting can facilitate good exposure of the posterolateral LV for inspection and mapping if necessary.14 Actual ablation, particularly cryoablation, is typically performed with cardiopulmonary bypass on an arrested heart to avoid the energy sink of warm blood within the heart. To access the LV endocardium, there are several options. The transaortic approach offers the best visualization of the basal LV, including the IVS region as well as the anterior and lateral LV walls.14,15 To achieve this, a standard aortotomy for aortic valve replacement is performed above the sinotubular junction.10 The aortic valve leaflets are carefully retracted against the sinus of Valsalva and the LV outflow tract is exposed (see Figure 129-3). The IVS is seen immediately under the right coronary aortic valve (AV) cusp. The basal anterior and lateral LV endocardium is visualized under the junction of right-left commissures and the left coronary cusp, respectively (see Figure 129-3). A transatrial, trans–mitral valve (MV) approach offers the best visualization of both papillary muscles, the posterior LV endocardium, and the LV apex.14,16,17 These structures are frequently involved in VT circuits in patients with healed inferior infarcts and can also be the source of VT in patients with nonischemic cardiomyopathy. In patients with mechanical AV and MV, apical ventriculotomy has been used to access the LV endocardium.18 In patients undergoing surgical ventricular aneurysm repair, the site of aneurysmectomy can also be used to access the LV endocardium before aneurysm resection and closure. The RV too can be accessed either via a transatrial, trans-tricuspid valve (TV) approach or directly through the RV free wall.14,18 An important difference between epicardial alone versus additional endocardial cardiac access pertains to cardiopulmonary bypass use.19 When the arrhythmia originates from the RV free wall or the inferior IVS region, access to these locations can be obtained by partial sternotomy, sparing the upper half of the sternum (see Figure 129-3).20 The purported advantages of this approach are shorter postprocedure recovery and more stable sternal healing. The authors have used this approach successfully in a patient undergoing ablation for refractory VT originating from the inferior IVS region and the anterior epicardial LV surface. There are also case series describing access to the epicardium using a surgical window via an epigastric incision after removal of the xyphoid process, or a left anterior thoracotomy using a limited left anterior incision extending from the third to fifth intercostal spaces. Both these techniques have been successfully accomplished in the cardiac electrophysiology (EP) laboratory. However, using this approach in the EP laboratory, only the epicardial aspect of the inferior, anterior, and parts of the lateral cardiac surfaces were accessed.21 Figure 129-3 Examples of different techniques to expose various cardiac surfaces during surgical VT ablation. A, Cardiac exposure via median sternotomy. B, Transaortic access to LV endocardium. C, Cardiac exposure via partial (inferior) sternotomy. In (A) and (C), the cryoprobe can be seen deployed at the LV apex and the inferior interventricular septal region, respectively. RV, right ventricle; LV, left ventricle; LCC, left coronary cusp; RCC, right coronary cusp; NCC, noncoronary cusp; AML, anterior mitral leaflet. Early attempts at mapping ventricular arrhythmias in the OR used finger-mounted bipolar electrodes that were manipulated to various locations in the chamber of interest. This method was adequate for identifying critical components of the reentrant circuit in patients with healed infarcts.3,4 Subsequently, multipolar meshes and basket catheters were developed, which had the ability to record electrical activity at a higher resolution than the finger-mounted bipolar electrode(s) and thus could provide more comprehensive understanding of the activation sequence during ventricular arrhythmias.22,23 However, interpretation of data required special signal processing, which made these tools cumbersome to use in the OR. With the development of electroanatomic mapping (EAM) techniques, electrophysiologists are now able to create a three dimensional shell of the chamber of interest during ongoing arrhythmia.24 This tool provides more intuitive visualization of the tachycardia circuit vis-à-vis the underlying anatomy and substrate. EAM requires a low-intensity magnetic field around the patient, which requires a special setup. In hybrid ORs that are specifically designed for both percutaneous catheter and open-heart surgical interventions, use of advanced cardiac EP mapping tools including EAM is possible.25 There are case reports describing the use of EAM in hybrid ORs during surgical VT ablation.11,18
Surgery for Ventricular Arrhythmias
Historical Perspective
Indications: Current and Emerging
Accessing the Ventricle: Approaches
Mapping: Tools and Techniques
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine
