CHAPTER 13 Surgery for Emphysema
The debilitating symptoms of pulmonary emphysema have attracted the interest of surgeons throughout the history of our specialty. Many innovative and creative operations have been devised to treat the dyspnea caused by this disease. Costochondrectomy, phrenic crush, pneumoperitoneum, pleural abrasion, lung denervation, and thoracoplasty all proved to be dead ends in the evolution of surgical treatment for the hyperexpanded and poorly perfused emphysematous lung.1 Only three surgical procedures have evolved to survive the test of time and to withstand the close scrutiny of the medical community: bullectomy, lung transplantation, and lung volume reduction. Bullectomy has roots dating to the first half of the last century, when external drainage of the giant bulla was attempted to eliminate the space-occupying lesion by collapse rather than by resection. Although vestiges of this conservative approach remain in use for rare high-risk patients, the general approach has evolved to include resection of the bulla with sparing of all functional lung tissue. Lung transplantation was successfully performed in 1963 by Hardy and coworkers,2 and after a prolonged period of incremental progress, the operation became clinically feasible in the early 1980s as heart-lung transplantation3 and isolated lung transplantation.4 Although lung transplantation was initially used as therapy for pulmonary fibrosis and pulmonary hypertension, the indications have evolved such that emphysema is the most common diagnosis leading to transplantation today. Lung volume reduction surgery (LVRS) was first proposed by Brantigan and colleagues5 in conjunction with lung denervation and was discarded after the initial experience; a mortality of 16% showed the operation to be too risky. Observations about the physiologic behavior of emphysema patients during and after lung transplantation led to the reconsideration of volume reduction by Cooper and associates.6
The destruction of pulmonary parenchyma causes a decreased mass of functioning lung tissue and thus decreases the amount of gas exchange that can take place. As the lung tissue is destroyed, it loses elastic recoil and expands in volume. This leads to the typical hyperexpanded chest seen in emphysema patients with flattened diaphragms, widened intercostal spaces, and horizontal ribs. These anatomic changes result in the loss of mechanical advantages exploited in normal breathing and thus lead to increased work of breathing and dyspnea.7 When the destruction and expansion occur in a nonuniform manner, the most affected lung tissue can expand to crowd the relatively spared lung tissue to impair ventilation of the functioning lung. Finally, there is obstruction in the small airways caused by a combination of reversible bronchospasm and irreversible loss of elastic recoil by adjacent lung parenchyma. The suitability of a given patient for surgical treatment of emphysema depends in part on the relative contributions of lung destruction, lung compression, and small airways obstruction to the overall physiologic impairment of that patient.
SELECTION OF PATIENTS FOR SURGICAL TREATMENT OF EMPHYSEMA
Bullectomy, lung transplantation, and LVRS are invasive procedures with risk of both morbidity and mortality to patients. Therefore, all three procedures are directed only at patients who remain symptomatic despite optimal medical therapy. This optimal medical treatment will include bronchodilators to eliminate any reversible component of airway obstruction. Smoking cessation is an absolute necessity and should be in effect for at least 6 months before surgical therapy is considered. Participation in pulmonary rehabilitation has been shown to relieve subjective dyspnea, to increase functional capabilities, and to improve subjective quality of life.8,9 All patients considered by the authors for surgical treatment of emphysema are enrolled in a supervised pulmonary rehabilitation program, and their subsequent consideration for surgery is based in part on their compliance and progress with rehabilitation. Finally, because the operations carry an immediate risk of morbidity and mortality, and because none has been shown to reliably increase life expectancy, patients considering an operation must be willing to accept the risks of surgery in exchange for an anticipated relief from dyspnea and an uncertain effect on life expectancy.
BULLECTOMY
Bullectomy is considered whenever a substantial air-filled bulla is detected on a chest radiograph. Most patients considered for surgery are symptomatic with dyspnea, pain, or spontaneous pneumothorax. Other symptoms are rare but include bleeding and infection within the confines of the bulla. The natural history of bullae treated expectantly with observation is one of enlargement causing worsened dyspnea, but the lack of large series of patients treated without surgery makes prediction of the rate of expansion unreliable. Some asymptomatic patients with a single bulla encompassing more than one half the volume of a pleural cavity would be considered surgical candidates, whereas patients with smaller lesions and no symptoms would be more controversial. Factors making surgery less appealing include multiple smaller bullae, advanced emphysema in the nonbullous adjacent lung, and notable comorbidities. The frequency with which bullectomy is performed is low, as demonstrated by a systematic review by Snider,10 who cited 22 individual reports during a 39-year period that included a total of 476 patients.
The safety of bullectomy in well-selected patients is demonstrated by the 2.3% mortality reported by FitzGerald and colleagues11 more than 30 years ago. Our modern results are similar, with a single death in 43 operations (2.3%).12 Because properly selected patients will have an increase in a first second forced expiratory volume (FEV1) postoperatively, there is a very low rate of respiratory failure and the need for tracheostomy. Parenchymal air leaks are the most frequent single postoperative complication, and they are suitably managed with the surgeon’s choice of buttressed stapled lines, pleural tent, pleurectomy, biological glues, or ambulatory Heimlich valves. In our series, 53% of patients experienced chest tube air leaks for more than 7 days.
There are few reports of long-term survival and functional changes after bullectomy, and none of them is a prospective clinical trial. Our clinical series demonstrated a 5-year survival of 91%, with two late deaths attributed to pneumonia and one to pulmonary fibrosis. In general, the freedom from long-term return of dyspnea is proportional to the quality of the remaining lung after bullectomy. All patients with emphysema seem to experience a progressive decline in FEV1 over time, so patients with nearly normal underlying lung at the time of bullectomy will begin at a higher functional baseline than patients with moderate or severe emphysema in the remaining lung. Our experience demonstrated an improvement in the FEV1 from 1.2 ± 0.6 L preoperatively to 1.9 ± 0.9 L at 6 months and 1 year postoperatively.12 The persistence of a measurable airflow obstruction after bullectomy underscores the presence of residual emphysema in the remaining lung, regardless of the normal appearance it may have compared with the destroyed bullous regions.
LUNG TRANSPLANTATION
Pulmonary emphysema was initially thought to be a contraindication for lung transplantation. In the era preceding bilateral lung transplantation, the perceived difficulty of ventilation-perfusion mismatching in the native and newly transplanted lung was thought to be an obstacle worth avoiding. For that reason, early isolated single-lung transplantations were directed at patients with pulmonary fibrosis, in whom the elevated pulmonary vascular resistance and poor compliance of the native lung created a situation in which the transplanted lung was both preferentially perfused and preferentially ventilated. After the initial success with single-lung transplantation for emphysema was reported,13 and after the development of techniques to allow safe, bilateral lung transplantation,14,15 the application of lung transplantation for emphysema quickly increased. Management of the recipient of a single lung transplant for emphysema has proved not to be as complicated as feared. The main principles that lead to success are the avoidance of positive end-expiratory pressure and the rapid weaning from mechanical ventilation.
Idiopathic emphysema and α1-antitrypsin deficiency together have become the most common indications for pulmonary transplantation. These two diagnoses together account for 63% of the adult single-lung transplantations and 32% of the bilateral lung transplantations reported in the 2003 Registry of the International Society for Heart and Lung Transplantation as reported by Trulock and colleagues.16
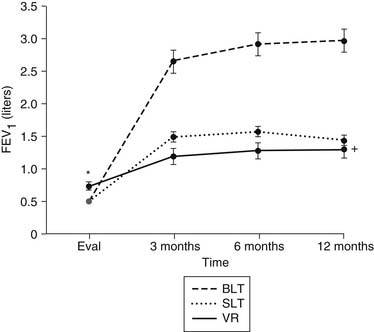
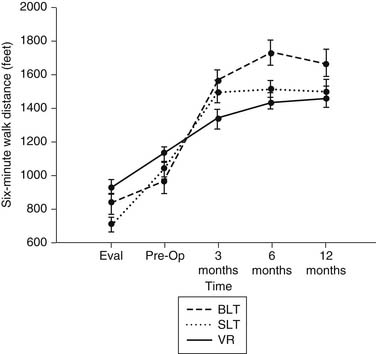
The advantage of lung transplantation is obvious: a complete replacement of the diseased and nonfunctional lung with a new and healthy donor lung. Initial and long-term function of patients with single or bilateral lung transplantation for emphysema shows a dramatic improvement in pulmonary function and exercise tolerance with elimination of the need for supplementary oxygen. Figure 13-1 demonstrates the magnitude of change in the FEV1 as a result of single-lung transplantation, bilateral lung transplantation, and LVRS as reported by Gaissert and colleagues.17 Figure 13-2 shows a similar stratified analysis for exercise tolerance as measured by 6-minute walking distances. It can be seen that although the improvement in both outcome measures is greatest for bilateral lung transplantation and least for LVRS, the absolute differences in exercise tolerance are much less than those seen in pulmonary function.
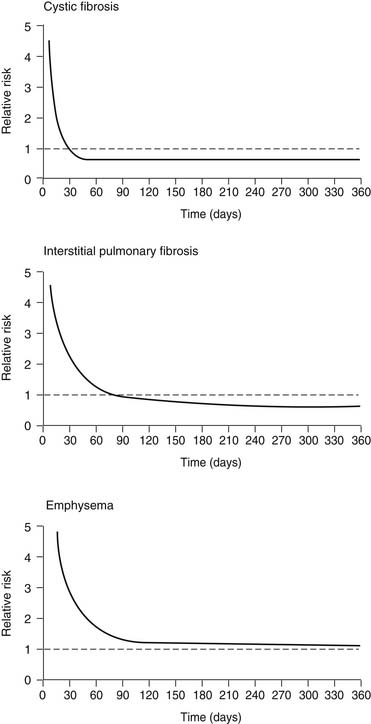
A controversial aspect of lung transplantation is the question of whether it conveys a survival benefit to the recipient. There have been no prospective randomized trials in which lung transplantation has been directly compared with medical therapy for treatment of advanced lung disease. As a result, the analysis of this question has been limited to the use of Cox regression analysis, with the transplantation procedure entered into the model as a time-dependent covariable. In essence, the patient’s time spent on the waiting list is used as the medical arm of the trial, and the survival after transplantation is used as the surgical arm. One such analysis by Hosenpud and colleagues18 showed the surprising finding that lung transplantation did not reduce the risk of death compared with the risk faced by remaining on the waiting list. This finding is graphically depicted in Figure 13-3. One explanation for this finding is the stable survival of emphysema patients on the waiting list and the unique situation in the United States that allows listing of patients for transplantation long before they are considered truly ready for such an operation. Other Cox regression models looking at this exact question have shown clear survival benefit for emphysema patients undergoing lung transplantation.19 More work in this field has been done, but it has been applied to lung transplantation in general and not to emphysema transplant recipients specifically.20–22
The choice of bilateral or unilateral transplantation for emphysema patients is controversial. In general, for younger patients, particularly those with α1-antitrypsin deficiency, we prefer bilateral sequential single-lung transplantation. The bilateral option is also more attractive in larger recipients who might never obtain a sufficiently large single-lung allograft. On the other hand, for smaller recipients, single-lung transplantation offers a suitable option, particularly when an oversized donor lung can be grafted. The earliest reports on the efficacy of lung transplantation for pulmonary emphysema compared the merits and risks of bilateral lung transplantation versus single-lung transplantation for these patients. The authors of these reports demonstrated a higher perioperative risk of the bilateral operation without a demonstrable functional benefit to the bilateral recipients.23,24 As a result, the single-lung transplantation quickly became the preferred operation for obstructive lung disease.
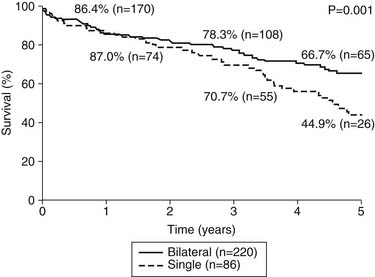
Our group recently reported a retrospective analysis of outcomes after lung transplantation in patients with chronic obstructive pulmonary disease (COPD).25 This report included 306 patients, 86 of whom received a single-lung transplant and 220 of whom received a bilateral transplant. In contrast to earlier reports from our institution, the morbidity and mortality were comparable for the two groups, with an overall hospital mortality of 6.2%. There were no differences in hospital stay, intensive care unit stay, or duration of mechanical ventilation. There was, however, a difference in long-term survival; the 5-year survival (Fig. 13-4) of the bilateral transplant recipients was 66.7%, whereas the survival of the single-lung recipients was 44.9%. The International Society for Heart and Lung Transplantation has reported a similar finding, but neither analysis is adjusted for other factors that might bias such a result.16 There is a likelihood that patients receiving single-lung transplants for emphysema might have a disproportionate share of risk factors, such as advanced recipient age and other comorbidities. Figure 13–4 shows the survival data for all lung transplantations performed by our group. As demonstrated, patients with COPD and α1-antitrypsin deficiency emphysema make up more than one half of our total recipients. It can also be seen that emphysema patients enjoy a better survival than do all other groups of patients, although the differences are not statistically significant. As a group, these COPD patients experience the best early outcomes after transplantation in most programs; the International Society for Heart and Lung Transplantation database cites a statistically significant 1.65 odds ratio of death in the first year after transplantation for patients with non-COPD diagnoses compared with those with COPD.16
Box 13–1 Indications and Contraindications for Lung Volume Reduction Surgery and Lung Transplantation
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree