Surgery for Coronary Disease
Surgery for revascularization of the myocardium continues to be an effective and lasting means of managing patients with multivessel coronary artery disease. However, the recent evolution of intracoronary stents has enabled interventional cardiologists to treat coronary stenoses percutaneously with early results approaching those of surgical bypass procedures. This has had an impact on the number and types of patients who are referred for coronary artery bypass surgery. Therefore, our surgical patients are now generally older, have more comorbid conditions, and more severe left ventricular dysfunction, and many have had previous catheter-based interventions. These patients are at increased surgical risk and may have poor surgical targets. To handle this group of patients, surgeons need to incorporate newer procedures into their practice, including off-pump surgery and transmyocardial laser revascularization, and pursue such strategies as angiogenic and cell-based technology.
Ultimately, the goal in the operating room is to provide patients with grafts that have the best long-term patency. The internal thoracic artery has proven to be the gold standard of conduits. Its patency is better than 90% at 15 years, and its use has been proven to prolong patient survival. The in situ left internal thoracic artery is the graft of choice to the left anterior descending (LAD) coronary artery. The in situ right internal thoracic artery has slightly lower patency compared with the in situ left internal thoracic artery. In younger patients, this is an excellent choice of graft to the ramus intermedius, proximal obtuse marginal coronary artery, or mid to distal right coronary artery. A free internal thoracic artery graft has a lower patency rate than an in situ graft.
If the right coronary artery is dominant and of good size, the right internal thoracic artery flow may be inadequate.
Bilateral internal thoracic arteries should be avoided in patients with insulin-dependent diabetes because of increased sternal complications.
Other arterial conduits have been used including the inferior epigastric artery, gastroepiploic artery, and radial artery. The inferior epigastric artery was found to have poor patency rates and is used rarely if at all. The gastroepiploic artery is still used by some surgeons, but harvesting this conduit requires entry into the peritoneal cavity and its use is therefore limited. The radial artery is now considered the second arterial conduit of choice (after internal thoracic arteries). One or both radial arteries can be used along with one or both internal thoracic arteries to provide complete arterial revascularization. The radial artery may be anastomosed proximally to the aorta, sewn in an end-to-side manner to an internal thoracic artery to create a Y graft, or sewn to the hood of a vein graft.
The greater saphenous vein has been extensively used as a conduit because it can be quickly procured, is easy to handle, and ensures excellent inflow. Renewed enthusiasm for this graft has been generated by the development of less invasive endoscopic veinharvesting techniques. The 10-year patency of greater saphenous vein grafts is 60% to 70%; however, this may be improved with the routine use of antiplatelet agents, cholesterol-lowering agents and angiotensinconverting enzyme inhibitors. Recent reports suggest that harvesting the saphenous vein with its surrounding tissue and not subjecting it to manual flushing or distension provide long-term patency rates comparable to those of the left internal thoracic artery.
Technique for Internal Thoracic Artery Harvest
The internal thoracic artery is a very delicate vessel that can be injured easily. Consequently, the artery should be dissected as a pedicle with great care.
A median sternotomy is made in the usual manner. The parietal pleura and pericardium are depressed gently, and the course of the internal thoracic artery is identified from its origin near the first rib to its termination beyond its bifurcation in the rectus sheath. A Favaloro
retractor provides excellent exposure. The Rultract System retractor also provides superb exposure and is probably less traumatic. The posterior rectus sheath is freed from the undersurface of the sternum and costal cartilages, allowing more extensive retraction with improved exposure of the internal thoracic artery.
retractor provides excellent exposure. The Rultract System retractor also provides superb exposure and is probably less traumatic. The posterior rectus sheath is freed from the undersurface of the sternum and costal cartilages, allowing more extensive retraction with improved exposure of the internal thoracic artery.
Overzealous elevation of the hemisternum by retractors may result in rib fractures or even costochondral disruption. This is more apt to occur in patients with pectus deformities or morbid obesity and in elderly patients with osteoporosis.
Although it is possible to dissect the internal thoracic artery without entering the pleural cavity, we prefer to routinely open the left pleura widely to provide excellent exposure and greatly facilitate harvesting of the internal thoracic artery.
 Opening the pleura allows the left internal thoracic artery pedicle to fall away from midline. This decreases the risk of injury at reoperation.
Opening the pleura allows the left internal thoracic artery pedicle to fall away from midline. This decreases the risk of injury at reoperation.Alternatively, the left internal thoracic artery can be harvested through a lower ministernotomy, dividing the left half of the sternum (see Chapter 1). A Favaloro retractor allows the left hemisternum to be elevated to provide adequate exposure for mobilizing the internal thoracic artery. This approach allows off-pump bypass grafting of the left internal thoracic artery to the LAD coronary artery (see subsequent text).
The internal thoracic artery is usually harvested as a pedicle with extensive use of electrocautery for chest wall hemostasis but not pedicle hemostasis. The parietal pleura on the internal intercostal musculofascial layer of the chest wall is incised approximately 7 to 10 mm medial to the internal thoracic artery along its entire course (Fig. 9-1). The blade of the electrocautery is then used to depress and dissect the pedicle from the chest wall. The lowest current is used to coagulate the internal thoracic artery and vein branches, well away from the parent trunks. The side branches on the artery are then occluded with fine metal clips. The pedicle is dissected from the level of the rectus sheath to the level of the subclavian vein where the artery passes beneath this vessel. Care is taken to identify and divide two intercostal branches, one passing anterior to the subclavian vein and a high first intercostal branch coursing laterally above the subclavian vein.
When the patient’s condition is unstable, it may be preferable to harvest the internal thoracic artery while the patient is on cardiopulmonary bypass.
Because the internal thoracic artery is a delicate structure, any undue stretching, clamping, or misplaced metal clips results in permanent vascular injury and therefore unsatisfactory short- and long-term results. Excessive traction during mobilization should be avoided as it can lead to dissection of the vessel wall.
When an electrocautery is used to divide the internal thoracic artery branches after application of metal clips, the heat and the electric current may conduct through the metal clip adjacent to the parent trunk and cause a burn injury. Therefore, branches must be
divided with scissors or coagulated well away from the metal clip adjacent to the internal thoracic artery.
divided with scissors or coagulated well away from the metal clip adjacent to the internal thoracic artery.
The internal thoracic artery pedicle must be dissected free from the chest wall along its entire course, from near its origin in the first intercostal space to well past its bifurcation into the rectus sheath to provide maximal length.
The first intercostal branch of the internal thoracic artery must be identified and divided to avoid any possible steal phenomenon from the internal thoracic flow.
Before commencing cardiopulmonary bypass, papaverine is sprayed gently over the pedicle and the adequacy of flow is determined. If there is no flow, a 1-mm vascular probe (Parsonnet) is cautiously introduced into the lumen of the vessel for a varying distance. This should be done with extreme care to avoid intimal injury. Usually very good flow is noted. Unless traumatized during harvesting, the internal thoracic artery usually provides adequate flow and should not be discarded.
 In elderly patients, it may be preferable to skeletonize the internal thoracic artery rather than harvesting it as a pedicle. This may decrease the incidence of avascular necrosis and infection affecting the sternum.
In elderly patients, it may be preferable to skeletonize the internal thoracic artery rather than harvesting it as a pedicle. This may decrease the incidence of avascular necrosis and infection affecting the sternum.The pedicle is laid on the heart to judge the appropriate length. The end of the pedicle is then grasped and occluded with forceps, allowing the internal thoracic artery to distend with blood. The artery is then cleaned free of the surrounding tissues, using sharp dissection. The artery is transected obliquely with the heel on the fascial side of the pedicle and prepared as a large hood orifice.
 The internal thoracic artery can be lengthened considerably with multiple pedicle fasciotomies. Maximal length can be obtained by skeletonizing the vessel along its course. Division of the internal thoracic veins should be avoided when performing the fasciotomies. If the pedicle remains too short, the artery may be divided proximally and used as a free graft.
The internal thoracic artery can be lengthened considerably with multiple pedicle fasciotomies. Maximal length can be obtained by skeletonizing the vessel along its course. Division of the internal thoracic veins should be avoided when performing the fasciotomies. If the pedicle remains too short, the artery may be divided proximally and used as a free graft.Excessive stretch and tension on the internal thoracic artery result in narrowing of the lumen and graft failure. This is seen as a string sign on a selective angiogram of the artery.
Before the distal end of the internal thoracic artery is divided, its correct length must be ascertained. The internal thoracic pedicle should lie very comfortably on the heart when it is full and the lungs are fully inflated; otherwise, the artery will be stretched and could become detached at the anastomotic site.
Similarly, it should not be redundant because too long a pedicle may curl or kink into the substernal area, increasing the risk of injury at reoperation.
The internal thoracic artery, when fully mobilized and divided, is occluded with an atraumatic bulldog clamp after the patient has been fully heparinized to prevent any clotting within the lumen of the vessel.
The pericardium is divided with electrocautery where the internal thoracic artery pedicle crosses it down to 1 cm above the left phrenic nerve. This allows the pedicle to assume a more lateral position and lie against the medial surface of the lung instead of coursing over the apex of the lung. This is especially important when the left internal thoracic artery is grafted to an obtuse marginal branch of the circumflex coronary artery.
An in situ right internal thoracic artery can easily reach the diagonal, ramus intermedius or a proximal obtuse marginal branch of the circumflex coronary artery. The pedicle should cross the distal ascending aorta near the innominate vein. Thymic tissue and fat can be used to cover the pedicle. If the right internal thoracic artery is grafted to the LAD coronary artery, its course will be across the more proximal aspect of the ascending aorta, which puts it at high risk of injury during reoperative procedures.
Technique for Radial Artery Harvest
Usually the nondominant arm is identified preoperatively for radial artery harvest. Intravenous catheters and
venipunctures are avoided in this arm. Allen test is performed using a Doppler probe to ensure adequate ulnar artery filling of the palmar arch. We usually perform preoperative assessment of radial arteries by ultrasound and Doppler for size and palmar arch patency.
venipunctures are avoided in this arm. Allen test is performed using a Doppler probe to ensure adequate ulnar artery filling of the palmar arch. We usually perform preoperative assessment of radial arteries by ultrasound and Doppler for size and palmar arch patency.
Open Radial Artery Harvest
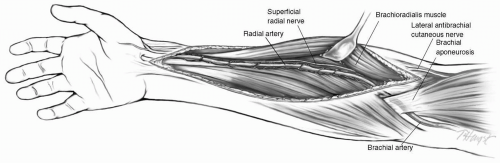
The arm is abducted to 90 degrees and under sterile conditions prepared and draped on an armboard. An incision is made in the midportion of the forearm over the belly of the brachioradialis muscle. The opening is then extended for a variable distance proximally toward the groove between the brachioradialis and biceps tendon (Fig. 9-2). Distally the incision is extended toward the wrist crease. With experience, the opening in the forearm can be fairly limited in length and still allow adequate exposure to harvest the radial artery.
The dissection of the radial artery begins distally by dividing the fascia and then proceeding proximally between the belly of the brachioradialis and flexor carpiradialis muscles. A vessel loop is then passed around the radial artery to facilitate exposure. The artery is dissected along with the two venae comitantes, double clipping and sharply dividing all the branches. When the radial artery is completely mobilized, the radial recurrent artery is identified proximally and the superficial palmar artery is seen distally. These two large branches define the limits of the dissection and should be preserved (Fig. 9-2). The radial artery is divided distally and proximally and placed in a solution of heparinized blood and papaverine. The arm incision is closed in two layers with continuous absorbable suture. The deep layer includes the subcutaneous tissue distally.
 The distal limit of the skin incision should be 3 cm above the wrist joint to decrease postoperative discomfort.
The distal limit of the skin incision should be 3 cm above the wrist joint to decrease postoperative discomfort.The distal portion of the radial artery tends to calcify. Unless the extra length is needed, the most distal segment of the artery should not be harvested and left in situ.
The superficial radial nerve provides cutaneous innervation to the radial aspect of the thumb and dorsum of the hand. It follows the middle third of the radial artery and is prone to injury. Similarly, excessive lateral retraction of the brachioradialis muscle may lead to injury to this nerve and resultant numbness of the thumb. This may occur in 5% to 10% of the patients undergoing radial artery harvest.
An electrocautery is used only on the skin and subcutaneous tissue. All the radial artery branches should be clipped proximally and distally with small metal clips before division. In addition, the proximal stump of the divided radial artery should be oversewn with a suture ligature to prevent late bleeding and hematoma formation.
The graft is gently flushed with heparinized blood and papaverine after harvesting. In addition, we routinely use intravenous nitrates or calcium channel blockers intraoperatively as well as postoperatively until the patient can tolerate oral medications. Most patients with free arterial grafts will be discharged with oral isosorbide dinitrates.
Compartment syndrome occurs rarely after radial artery harvest. However, if not recognized and treated promptly, extensive muscle loss and distal ischemic injury can occur. The hand must be checked for unrestricted movement and intact sensation at routine intervals in the immediate postoperative period to prevent such devastating consequences.
The distal end of the radial artery is dissected free of the accompanying veins and surrounding tissue. An oblique opening is created, which can be enlarged to match the coronary artery opening by making a short longitudinal incision at the heel.
Endoscopic Radial Artery Harvest
In the operating room, the arm is abducted to 90 degrees. Under sterile conditions, the arm is prepared and draped on an armboard. A sterile blood pressure cuff is placed around the upper arm and connected to a sphygnomanometer. In addition, a small roll is placed under the wrist to hyperextend the hand. A 1-in. longitudinal incision is made over the radial artery distally, such that it will be covered with the sleeve of a shirt (Fig. 9-3A). Under direct vision, the radial artery is dissected for a short distance proximally and distally. The blood pressure cuff is inflated to 20 mm above the patient’s systolic blood pressure. The endoscope is inserted through the incision while the tunnel is being insufflated. The tissue plane under the radial artery is dissected first, followed by circumferential mobilization. The branches are freed gently for a sufficient length so that cautery can be applied without injury to the radial artery wall. The dissector is then replaced with scissors equipped with cautery to divide all side branches (Fig. 9-3B). A counter incision at the elbow is made, and the proximal end of the radial artery is divided and controlled. Alternatively, an endoloop can be advanced through the tunnel and proximal control can be obtained. The latter technique avoids the counter incision. After removal of the radial artery from the tunnel, metal clips are applied to the branches (Fig. 9-3C). The distal radial artery is ligated and divided. Under endoscopic visualization, the blood pressure cuff is deflated and optimal hemostasis is ensured. The wrist incision is closed in two layers and a sterile pressure dressing is applied. The radial artery is irrigated with heparinized saline. Papaverine may be sprayed on the pedicle to prevent spasm.
Techniques for Greater Saphenous Vein Harvest
Traditional open vein harvesting through one long incision or multiple interrupted incisions can result in significant wound morbidity including infection and chronic leg edema. The endoscopic approach avoids the healing problems associated with a long leg incision and may, in particular, benefit patients with diabetes, obesity, or peripheral vascular disease.
Endoscopic Saphenous Vein Harvest
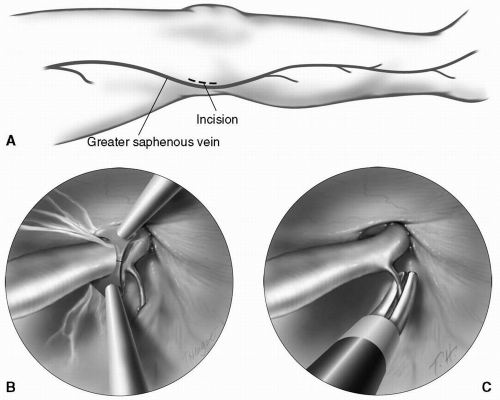
A 2-cm transverse incision is made just above the medial aspect of the knee (Fig. 9-4A). The greater saphenous vein is identified and encircled with a vessel loop. An endoscope is then introduced using carbon dioxide insufflation to dissect a plane superficial to the vein. Circumferential dissection of the vein is completed by the dissector. The side branches are dissected for a minimum of 5 mm, so
that with cauterization, the vein wall is not damaged (Fig. 9-4B). After removal of the dissector, a specialized cautery device/scissors is introduced to divide the side branches (Fig. 9-4C). Once the proximal extent of the dissection is reached, a counterincision is made to facilitate division of the vein and oversewing of the stump. The vein is then gently withdrawn through the knee incision.
that with cauterization, the vein wall is not damaged (Fig. 9-4B). After removal of the dissector, a specialized cautery device/scissors is introduced to divide the side branches (Fig. 9-4C). Once the proximal extent of the dissection is reached, a counterincision is made to facilitate division of the vein and oversewing of the stump. The vein is then gently withdrawn through the knee incision.
If two vein graft segments are required, the thigh vein is harvested as described. For additional conduit, the lower leg vein can be harvested by directing the endoscope inferiorly through the same knee incision.
The two incisions are then closed in two layers, and an elastic bandage is snugly wrapped around the leg and kept in place for 24 hours.
Endoscopic dissection with carbon dioxide insufflation causes vein compression and can lead to stasis of blood flow. Heparin should be administered intravenously before endoscopic vein harvest to prevent intraluminal thrombus formation.
Hemostasis must be meticulously achieved using an electrocautery to avoid the development of a hematoma. If a large area of dead space has been created by the dissection, a soft drain connected to a closed drainage system should be placed along the endoscopic tract and left in place for 24 hours.
Excessive traction on the vein to mobilize it and withdraw it from the endoscopic tract can lead to intimal injury and avulsed branches. This must be avoided by extensive dissection with the cautery device and endoscopic scissors.
The learning curve for using endoscopic equipment is significant. With experience, it adds little time to the overall operation. Endoscopic vein harvesting is our procedure of choice when two or more vein conduits are required.
Open Greater Saphenous Vein Harvest
An incision is made in the groin, one fingerbreadth medial to the femoral artery pulse. The subcutaneous tissue is dissected to expose the greater saphenous vein as it curves to pierce the cribriform fascia of the femoral sheath and join the femoral vein. The skin incision is then extended downward along the course of the vein. The incision can alternatively be started at the ankle, anterior to the medial malleolus, and extended upward. Many surgeons consider this approach convenient and elect to use it routinely.
The vein is harvested using the “no touch” technique. This entails handling of the vein only by its adventitia with an atraumatic vascular forceps. The vein is then gently removed from its bed by careful dissection and division of its branches.
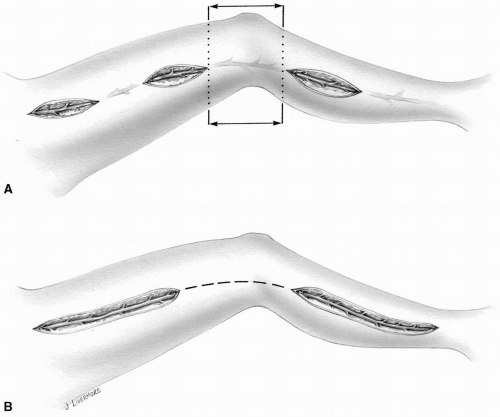 FIG 9-5. Open harvest of a greater saphenous vein. A: Multiple skin incisions. B: Long incisions leaving a skin bridge alongside the knee joint. |
Harvesting veins from limbs with evidence of infection or ulceration should be avoided if possible.
With the aid of a pair of sharp scissors, the skin incision is extended over the surgeon’s index finger, which has tunneled above and parallel to the saphenous vein. This technique prevents accidental division of a more superficially placed saphenous vein and eliminates the development of unnecessary dead spaces or redundant skin flaps.
The saphenous nerve runs a course along the greater saphenous vein. Special care should be taken not to divide it to avoid postoperative paresthesia.
The incision alongside the knee joint is subjected to much strain and stretch in several directions as the joint moves. This may give the patient significant discomfort and interferes with satisfactory healing. Therefore, the skin in this location is usually left intact (Fig. 9-5).
In patients who are diabetic or have peripheral vascular disease and are prone to poor wound healing, multiple skin incisions are made, leaving intervening bridges of skin intact. This allows better closure of the wound and minimizes ischemic changes along the skin edges (Fig. 9-5A).
A wound in the lower leg tends to heal slowly; this is of particular significance in the elderly diabetic patient with peripheral vascular disease. Meticulous handling of tissues and careful wound closure are mandatory.
 It is perhaps preferable not to harvest veins from the lower legs of elderly patients with diabetes or peripheral vascular disease.
It is perhaps preferable not to harvest veins from the lower legs of elderly patients with diabetes or peripheral vascular disease.When both greater saphenous veins have been stripped for varicosities or removed for previous bypass procedures, a search should be made for one or both lesser saphenous systems. Often, an adequate segment of vein can be procured. In these cases, the patient should be prepared and draped in such a manner that the back of the legs can be exposed. Cryogenically preserved homograft vein grafts
are available in tissue banks in most cardiac surgery centers and can provide an alternative when no other autologous vein or arterial conduit is available. The long-term patency of these grafts is poor compared with that of native vein conduits.
are available in tissue banks in most cardiac surgery centers and can provide an alternative when no other autologous vein or arterial conduit is available. The long-term patency of these grafts is poor compared with that of native vein conduits.
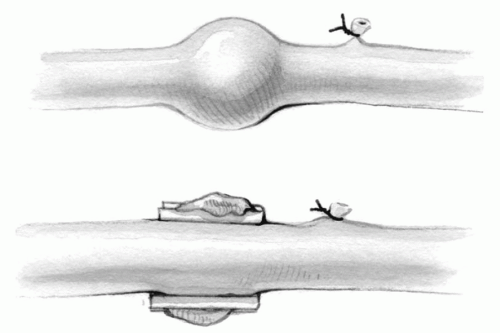
Saphenous veins with varicosities should be avoided. The walls of these vessels are dilated and abnormal, and the large caliber predisposes to lower flow velocity and possibly early graft thrombosis and occlusion.
Localized varicosities can be detected along the vein wall when it is being gently distended. They may be partially excluded by the application of metal clips on the redundant tissue parallel to the vein wall (Fig. 9-6).
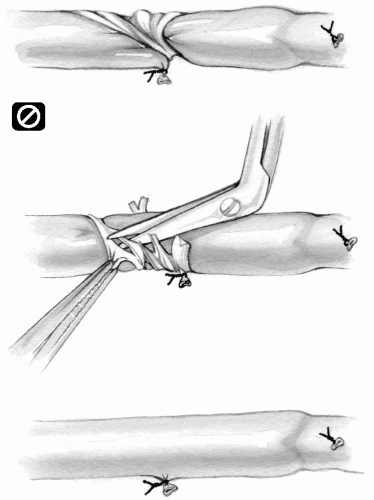
The vein must never be pulled or stretched to facilitate dissection. The intimal layer is very delicate and may tear, giving rise to the formation of a nidus for platelet aggregation and possible subsequent early occlusion of the graft (Fig. 9-7A). This is more likely to occur when multiple skin incisions are made and the vein has to be harvested from beneath the skin bridges.
The vein graft should be gently distended; any excessive pressure can result in intimal tear and disruption. Devices are commercially available to prevent the intraluminal pressure from exceeding 150 mm Hg.
Stretching of the vein may also result in avulsion injury owing to tension on small side branches. These tears on the vein wall can be oversewn with 7-0 or 8-0 Prolene sutures to ensure adequate hemostasis; however, the vein integrity remains disrupted.
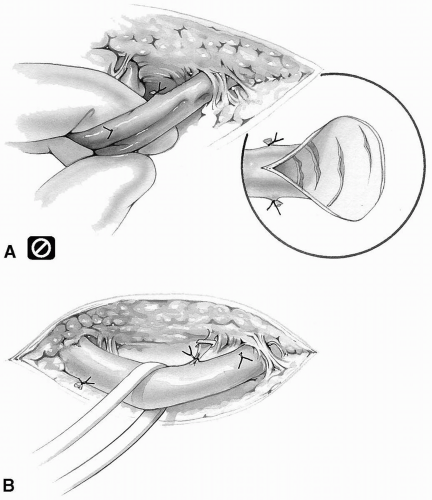 FIG 9-7. A: Pulling or stretching vein injures the intima. B: Gentle retraction with an elastic band. |
The vein can be gently retracted by means of elastic vessel bands whenever necessary (Fig. 9-7B).
The side branches are identified and ligated; alternatively, they can be occluded with metal clips and then divided (Fig. 9-8).
The branches should be ligated or clamped approximately 1 mm from the vein wall to minimize the presence of a stump, which may predispose to thrombus formation and early graft occlusion (Fig. 9-9A). Any stump can easily be eliminated by application of a fine metal clip behind the tie, parallel with the vein wall (Fig. 9-9B).
Conversely, the tie or metal clip should never occlude part of the vein wall itself. This gives rise to localized constriction (Fig. 9-9C). The tie or clip should be gently removed. Applying pressure with a heavy needle driver on the closed loop of the metal clip will separate the two ends and facilitate its removal. The tie or metal clip is replaced or reapplied appropriately.
The adventitial tissue may at times be caught in the tie around one of the branches, creating a localized
constriction. The adventitial band should be carefully divided with Potts scissors (Fig. 9-10).
constriction. The adventitial band should be carefully divided with Potts scissors (Fig. 9-10).
When an adequate segment of vein is dissected free, it is divided at each end and removed. The vein stumps in the groin and the ankle are securely ligated.
Skin Closure
The leg wound is closed in layers with absorbable sutures. In the groin region or where the wound is deep, an extra layer of closure may be necessary. The skin is closed with fine absorbable suture material in a subcuticular manner.
If the wound is deep or continues to ooze blood, closed-system drainage for 24 hours should be used. This prevents hematoma formation and possible infection.
Patients with diabetes and patients with peripheral vascular disease are at increased risk of this complication. Therefore, the wound must be closed atraumatically and without leaving any dead space. Absolute hemostasis must be achieved before closure is begun. The subcuticular skin closure may be reinforced with deeply placed, interrupted horizontal mattress monofilament sutures that are left in place until satisfactory healing has been completed, usually for at least 2 to 3 weeks.
Regardless of harvest technique, an olive-tipped cannula is introduced into the distal end of the vein. The vein is gently distended with autologous heparinized blood. Any avulsed branches are identified and securely ligated with 4-0 silk or oversewn with 7-0 or 8-0 Prolene sutures, taking all the aforementioned precautions into consideration (Fig. 9-11).
At times, the wall of the vein itself at the site of the avulsion of its branches requires suture closure; this can be accomplished by taking longitudinal bites of the vein wall with 7-0 or 8-0 Prolene when it is being distended. Transverse suturing gives rise to localized constriction (Fig. 9-12).
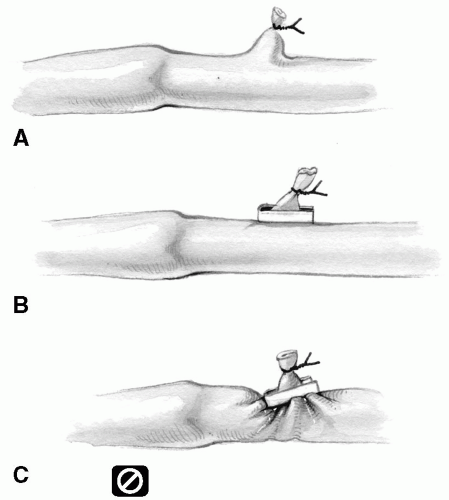 FIG 9-9. A: Leaving excess stump on a vein branch. B: A metal clip eliminates stump. C: A clip constricting vein. |
The end of the vein is then cut, avoiding any intimal valvular remnant, and trimmed so that is has a smooth, hood-shaped orifice for anastomosis to the coronary artery (Fig. 9-13).
If the caliber of the vein is small, the opening may be further enlarged by incising the vein orifice at the heel.
Some surgeons have advocated the use of a valvulotome to cut away the valve leaflets in the saphenous veins. Although this appears to be useful at times, it may create buttonhole defects in the vein wall. Therefore, if the device is used, great caution must be exercised. We do not routinely remove the valve leaflets unless they are located at the anastomotic sites.
Traditionally, the vein procured from the lower leg conforms more to the caliber of the coronary arteries, has few if any valves, and can withstand higher intraluminal pressure. It is therefore more suitable for bypass grafting of the smaller coronary arteries. However, the normal arterialization process and intimal hyperplasia may result in higher early graft closure of small saphenous vein conduit. Despite all its advantages, the proximal end of a narrow-caliber vein graft may be too small for a standard aortic anastomosis. The proximal anastomosis will have to be carried out to a smaller aortic opening rather than a regular punched-out hole.
Coronary Artery Bypass Grafting with Cardiopulmonary Bypass
Although many approaches have been used in the last decade for coronary artery bypass surgery, including limited thoracotomy incisions and endoscopic techniques, a
median sternotomy is considered the incision of choice in most cases today. A significant number of coronary bypass cases is now being performed without the use of cardiopulmonary bypass (see subsequent text). However, cardiopulmonary bypass is often preferred or required.
median sternotomy is considered the incision of choice in most cases today. A significant number of coronary bypass cases is now being performed without the use of cardiopulmonary bypass (see subsequent text). However, cardiopulmonary bypass is often preferred or required.
Venous drainage is accomplished through a single atriocaval cannula in most patients undergoing coronary artery bypass surgery. Bicaval cannulation is used when concomitant procedures necessitating an opening into the right side of the heart are indicated. Oxygenated blood is returned to the patient by direct cannulation of the ascending aorta. In rare instances when aortic cannulation is not feasible because of an ascending aortic aneurysm or extensive aortic wall calcification, the femoral or axillary arterial route is chosen instead (see Chapter 2).
Venting of the left side of the heart through the right superior pulmonary vein or through the pulmonary artery has been used, but it is unnecessary in most instances (see Chapter 4). In rare instances, when reoperative surgery for a single bypass to the circumflex coronary artery is needed, a left thoracotomy is an alternate approach. In such cases, cardiopulmonary bypass is achieved by both femoral artery and femoral vein cannulation (see technique described in Chapter 2).
Myocardial Preservation
Cold blood cardioplegia is infused into the aortic root to achieve cardioplegic arrest of the heart initially and repeated every 10 to 15 minutes during the cross-clamp time. Additional cardioplegic solution is infused directly into the vein graft after the distal anastomosis is completed. Core cooling to 34°C and topical cold or iced saline supplement myocardial protection. Critical proximal disease of major coronary arteries may interfere with the uniform distribution of cardioplegia and prevent complete cardioplegic arrest of the myocardium. Retrograde cardioplegic perfusion through a coronary sinus catheter is a useful adjunct for optimal myocardial protection during coronary revascularization (see Chapter 3).
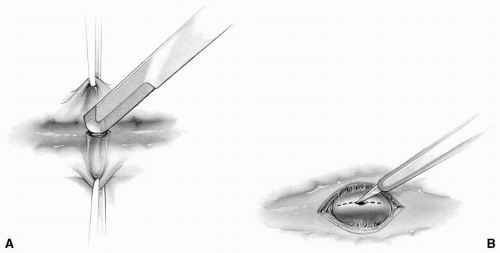 FIG 9-14. A: A stroker blade is used to expose coronary artery. B: A poker blade is used to incise the anterior wall of a coronary artery. |
In patients with acute coronary occlusion and impending infarction, the culprit vessel is grafted first to allow cardioplegic solution to be delivered through the vein graft to the involved myocardial territory.
 Retrograde administration of cardioplegia may be particularly useful when arterial conduits are used because cardioplegia cannot be delivered through the graft.
Retrograde administration of cardioplegia may be particularly useful when arterial conduits are used because cardioplegia cannot be delivered through the graft. Patients undergoing redo coronary artery bypass procedures with patent but diseased vein grafts are at risk of embolization of graft debris into the distal coronary artery bed. In these cases, retrograde cardioplegia is indicated.
Patients undergoing redo coronary artery bypass procedures with patent but diseased vein grafts are at risk of embolization of graft debris into the distal coronary artery bed. In these cases, retrograde cardioplegia is indicated.Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree