CHAPTER 123 Surgery for Congenital Anomalies of the Aortic Valve and Root
Surgical Anatomy of the Aortic Valve and Root
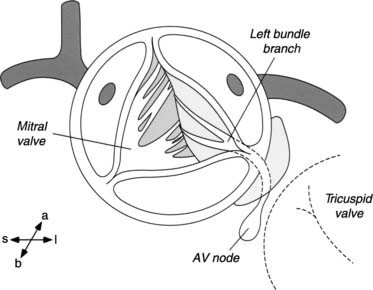
The subvalvular anatomy is dominated by the anatomic relationships between the aortic valve, the interventricular septum, the membranous septum, the mitral valve, and the conduction apparatus (Fig. 123-1). Parts of the aortic leaflets are in fibrous continuity with the anterior leaflet of the mitral valve as well as the tricuspid valve (via the membranous septum). These structures are the fibrous skeleton of the heart, and they are its central supporting structure. In addition to providing points of fixation for the atrioventricular valves, the fibrous skeleton also provides electrical insulation between the atria and the ventricles, restricting impulse conduction to the bundle of His. After arising from the atrioventricular node, the bundle of His penetrates the membranous septum, emerging on the surface of the left ventricular septum immediately below the aortic anulus. From a view through the aortic valve, the bundle lies beneath the anulus, just below the commissure between the noncoronary and right coronary leaflet. From the surgeon’s perspective, important radiations of the bundle reach to the nadir of the right coronary leaflet as they fall away toward the apex of the heart.
The aortic sinuses are dilations of the aortic wall above, distal to the insertion of the semilunar leaflets, and they are well suited to support the coronary ostia. While the leaflets retract during systole, eddy currents developing in the sinuses prevent occlusion of the coronary ostia by the retracted aortic leaflets. Their presence is probably important to the long-term function of the aortic valve leaflets.1,2 Finally, the supravalvular area denotes the transition from the left ventricle–aorta complex to the aorta proper. For practical purposes, this area includes the sinotubular junction, the area just distal to the tips of the commissural posts and the dilations of the aortic sinuses.
AORTIC STENOSIS
Prevalence
Congenital aortic stenosis has been reported to be present in between 3% and 6% of children with congenital heart disease.3 Males are affected more commonly than females with an incidence of 3:1.4 Although aortic insufficiency may afflict congenitally abnormal valves later in life, it is decidedly uncommon during infancy and early childhood. When present in these age groups, it is usually an iatrogenic consequence of a procedure designed to relieve congenital aortic stenosis.
Diagnosis
Echocardiography
Echocardiography is the mainstay for contemporary diagnosis of congenital aortic valve disease. This noninvasive test provides important anatomic and physiologic information, such as the number and anatomy of the aortic leaflets, the size of the aortic anulus and ascending aorta, the location of the coronary arteries, the adequacy of the subaortic LVOT, and the site of the hemodynamic stenosis. The peak instantaneous pressure gradient may be estimated by measuring the velocity of blood flow across the stenosis and employing a modification of the Bernoulli equation (velocity [m/s]2 × 4).5
Recent developments in imaging include the availability of three-dimensional (3D) echocardiographic technology. Ventricular mass and volume measurements obtained by 3D echocardiography compare well to those obtained by magnetic resonance imaging, and its value in determining the etiology of congenital aortic valvular disease is currently under investigation.6
Stress Testing
Stress testing may be helpful in older patients with mild or moderate aortic stenosis when symptoms are vague or suspect and not clearly related to the aortic valve disease. Stress-related changes in the S-T segment or in T-wave morphology would suggest important stenosis with myocardium at jeopardy. In these instances, relief of the stenosis should be seriously considered, despite mild or moderate resting peak gradients.
Natural History
Aortic valve disease in children tends to be progressive, with stenosis increasing over time, and it can be classified as mild, moderate, or severe. As categorized by Hossak and colleagues,7 patients with mild stenosis have normal pulse volumes with a resting peak systolic gradient (measured at catheterization) between the left ventricle and the aorta of less than 40 mm Hg. Patients who present with moderate aortic stenosis have diminished pulse volumes by palpation and resting peak systolic gradients of 40 to 75 mm Hg at rest. Patients who present with severe stenosis have abnormal pulse volumes and a resting peak systolic pressure gradient in excess of 75 mm Hg. These gradient criteria were obtained by direct pullback measurements obtained at catheterization. Currently, gradients across the LVOT are most often obtained by echocardiography. In most cases, Doppler-derived peak instantaneous gradient correlates well with catheter-derived data, but at lower gradients, Doppler may result in an overestimation.8,9
Because aortic valve disease in children is a progressive disease, the natural history of this progression is of some interest. Mills and coworkers10 reported that 7% of children presenting with nonobstructive aortic lesions progressed to mild obstruction after 7 to 15 years.
When mild stenosis was seen on the initial evaluation, progression is more rapid. Twenty percent of patients develop moderate or severe stenosis within 10 years and 45% progress within 20 years; about 60% of patients presenting with moderate stenosis progress to severe stenosis within 10 years.7
Treatment
Critical Aortic Stenosis in the Neonate
Aortic stenosis seen in the neonatal period may be a very severe hemodynamic lesion that is life-threatening (Fig. 123-2). These children often present in shock as a consequence of ductal closure in the setting of left heart structures that are inadequate to support the systemic circulation; their systemic circulation is ductal dependent. These children require urgent medical stabilization, including assisted ventilation and inotropic support. Prostaglandin E1 is administered to reopen the duct and to maintain ductal patency. Once the child is stabilized, a thorough echocardiographic examination of the structures on the left side is required to determine their suitability to support the systemic circulation.
When approaching critical aortic stenosis in the neonate, the single most important decision regarding treatment is to decide if the patient will benefit from biventricular repair on if the patient is better suited to a single-ventricle approach. The importance of proper treatment selection is reflected by the high mortality in older unstratified series of neonates undergoing valvotomy for critical aortic stenosis.11 An accurate determination of which patients will benefit from a single-ventricle treatment pathway (Norwood operation) is critical and has been the impetus for the study sponsored by the Congenital Heart Surgeons Society (CHSS) and designed to accomplish this.12 An estimate of survival benefit for specific left-heart morphology can be obtained at the CHSS website (www.chssdc.org). The management of these patients has been detailed elsewhere. This discussion focuses on patients with aortic stenosis as the dominant lesion in the setting of two adequate ventricles.
For patients with anatomy deemed suitable to support a two-ventricle circulation, aortic valvotomy provides very effective relief of aortic stenosis. Two techniques of aortic valvotomy have been developed: balloon dilation of the stenotic valve and surgical valvotomy (open and closed). Although balloon valvotomy is the favored technique at many institutions, surgical valvotomy is preferred by some. Proponents of balloon valvotomy cite avoidance of potential surgical morbidity, whereas advocates of surgical valvotomy maintain that a more accurate valvotomy is possible under direct vision.
Although there are no prospective randomized studies directly comparing these two techniques, some data are available. McCrindle and colleagues13 reported a CHSS-sponsored multi-institutional review of 110 neonates undergoing either surgical (n = 28) or balloon (n = 82) valvotomy for critical aortic valve stenosis in the neonatal period. Survival was similar between the two procedures (82% at 1 month, 72% at 5 years). Although balloon valvotomy was more effective at relieving stenosis (mean residual gradient, 20 versus 36 mm Hg), it was accomplished at the expense of a higher incidence of important aortic insufficiency (18% after balloon valvotomy versus 3% after surgical valvotomy). Despite these differences, the outcome data between the two techniques are quite comparable. Overall freedom from re-intervention was similar for both groups (91% at 1 month, 48% at 5 years). The need for subsequent procedures, regardless of technique, emphasizes the palliative nature of valvotomy for critical aortic stenosis.
McElhinney and colleagues14 reported medium- and long-term follow-up of 113 patients (age, 60 days) from Children’s Hospital Boston performed between 1985 and 2002. They reported a normalization of aortic anular and LV end-diastolic dimensions within 1 to 2 years. Freedom from moderate or severe aortic regurgitation was 65%, with a re-intervention–free survival of 48% at 5 years. These data suggest that early relief of the LVOT obstruction allows catch-up growth without neonatal surgery.
Although balloon valvotomy has assumed a prominent role in the treatment of congenital aortic stenosis in many institutions, its role vis-à-vis surgical valvotomy remains controversial. Hawkins and colleagues15 have estimated the incidence of aortic valve operation after balloon valvotomy to be 5% to 7% per year. Others have reported the risk of surgery to be lower, but the incidence of re-intervention, including subsequent balloon dilation, remains high (60% at 8 years).16 It may be, however, that specific aortic valvular substrates lend themselves to one treatment or the other. In a group of 54 infants (57% neonates) undergoing surgical aortic valvotomy, Bhabra and coworkers17 reported significant differences in the long-term outcomes based on leaflet morphology. When valvotomy resulted in a trileaflet structure, patients did significantly better than when only a bileaflet valve was achieved. At 10 years, the actuarial freedom from re-intervention was 92% among patients with trileaflet valves but only 33% among those with bileaflet valves (P = .01). Similar differences were reported for freedom from aortic valve reoperation (P = .04). Freedom from aortic valve replacement was 100% for those with trileaflet valves and 57% for those with bileaflet valves. However, by echocardiogram, the authors were able to retrospectively identify only 14 of 28 bileaflet valves, whereas seven of eight valves with trileaflet potential could be identified (sensitivity, 88%; specificity, 50%.). These results have yet to be confirmed, but closer examination of the anatomic subtypes of aortic valve stenosis may be justified before selecting the appropriate technique.
There appears to be little if any role for surgical transventricular (closed) aortic valvotomy.
Catheter versus Surgical Relief in Infancy
A few studies have compared balloon aortic valvotomy to surgical valvotomy in older children. McCrindle18 reported an analysis of 630 balloon valvotomies on 606 patients from 23 institutions. The median age was 6.8 years, with a range of 1 day to18 years. Because of technical issues, the procedure was abandoned 4.1% of the time, and procedural mortality was 1.9%. A suboptimal result (failure to complete procedure, residual gradient >60 mm Hg, left-ventricle-to-aortic pressure ⬧1.6, or major morbidity or mortality) was reported in 17%. Independent risk factors for poor outcomes were age less than 3 months, earlier procedure date, higher preoperative gradient, unrepaired aortic coarctation, and the use of undersized balloons.
Other groups have reported similar results with balloon valvotomy in non-neonates. Moore and colleagues16 reported successful dilation in 87% patients (129/148), with a very low procedural mortality (0.7%) and good long-term survival (95% at 8 years). Freedom from re-intervention at 8 years was 50%, which is consistent with other studies.19 In these patients, the risk of repeat intervention was related to the degree of regurgitation and to residual gradients after initial balloon valvotomy. As in the report by Bhabra and coworkers,17 Moore and associates16 reported differential results based on angiographic morphology of the stenotic aortic valve.
However, as in non-neonates, it is difficult to clearly demonstrate superiority of balloon or surgical aortic valvotomy. Chartrand and colleagues20 reported their experience with 67 children (older than 6 months) undergoing surgical valvotomy between 1960 and 1992. There was no operative mortality, and the percentages for 20-year freedom from death, reoperation, and aortic valve replacement were 94%, 63%, and 73%, respectively. The authors concluded that surgical valvuloplasty is a safe and effective procedure with durable results. In summary, as in neonates, aortic valve morphology appears to influence the response to intervention, and further characterization may be justified. It appears that institutional preference for balloon or surgical valvotomy can be defended on the basis of experience.
Aortic Stenosis in the Older Child
In contrast to neonates, who present clinically with critical aortic stenosis, older children are commonly asymptomatic. For them, durable preservation of LV function becomes the primary goal of treatment. The etiology of aortic stenosis in the older child (>1 year) is most commonly valvular aortic stenosis (79%), with subvalvular aortic stenosis (7%) and supravalvular stenosis (6%) being much less common.4 When it is present, aortic regurgitation is often associated with congenital aortic stenosis and may be the consequence of previous interventions for relief of stenosis.
Most older children with aortic stenosis enjoy normal growth and development. Symptoms such as chest pain, exercise intolerance, or syncope constitute clear surgical indications. For asymptomatic patients, the indications for intervention are more subtle. For patients with severe stenosis (left-ventricle-to-aortic gradient >75 mm Hg), operation is recommended. For children thought to have moderate stenosis (40 to 75 mm Hg gradient), more information may be needed before a recommendation can be made. In this setting, electrocardiographic changes (ST-T-wave changes consistent with left ventricle strain, left ventricle hypertrophy) or a positive stress test are indications for operation. For these asymptomatic patients, somatic growth, surgical options, and timing of intervention become important concerns.
SUBVALVULAR AORTIC STENOSIS
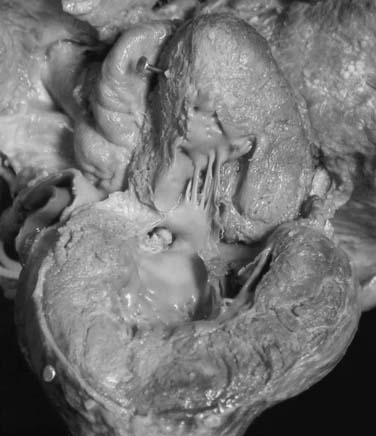
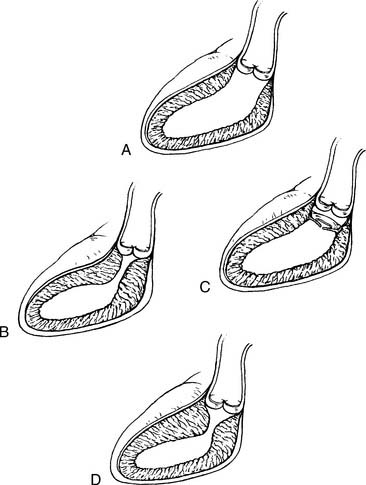
Fixed subaortic stenosis is a heterogeneous lesion that can take the form of a discrete membrane, tunnel-like stenosis, or muscular obstruction (Fig. 123-3). A variety of surgical approaches have been designed to address each of these variants.
Membranous Subaortic Obstruction
This lesion is found in association with other congenital lesions in 60% to 70% of cases, with a ventricular septal defect (VSD) being the most common (35%).21 Membranous subaortic stenosis results from the proliferation of fibrous tissue just beneath the leaflets of the aortic valve. This tissue may be very thin and tough, and it is often circumferential, involving the underside of the anterior leaflet of the mitral valve. In fact, careful examination of the echocardiogram may suggest a hinge point representing tethering on the leaflet by the membrane.22,23 Although the etiology of this form of aortic pathology is not known, studies have suggested that shear stress, precipitated by abnormal angles between the ventricular septum and the aortic barrel, may play an important role. The addition of a VSD adds to the generation of shear stress in this setting.24
Surgical Indications
In general, the surgical indications for membranous subaortic stenosis adhere to the general recommendations for aortic stenosis. However, citing a lower incidence of recurrence, some centers advocate earlier surgical resection for subaortic stenosis. Brauner and coworkers25 reported that the recurrence rate of subaortic stenosis (predominantly composed of membranous stenosis, but with a few tunnel-like stenoses) was related to a preoperative gradient of 40 mm Hg or greater, and they argued that surgical resection at lower gradients was justified. Some groups have advocated repair at the time of diagnosis, regardless of the gradient.26,27 However, the advantages of early intervention have not been confirmed, and other investigators have reported no benefit of early surgery on recurrence.28
Because the timing of surgery is controversial, it is helpful to examine the natural history of membranous subaortic stenosis. Some data suggest that some children with mild subaortic stenosis (peak systolic gradient <40 mm Hg) may not require surgery for at least several years. A large representative study of the rate of progression of subvalvular aortic stenosis was reported by Rohlicek and coworkers29 studying children from several centers in eastern Canada. To document the natural history and surgical outcomes, they followed 92 children from the time of diagnosis. There was a slight male preponderance (1.6:1), and the mean age at diagnosis was 5.3 years. Thirteen patients had bicuspid aortic valves. Forty-two children ultimately came to surgery an average of 2.2 ± 0.4 years after diagnosis; 44 were followed medically and never came to operation (mean follow-up, 4 years). Children ultimately requiring surgery presented with higher initial gradients (40 ± 5 versus 21 ± 2 mm Hg), and were more likely to present with aortic insufficiency at diagnosis (35% versus 13%). Analysis showed the echogradient at diagnosis to be predictive of subsequent gradient progression as well as the appearance of aortic insufficiency. Eight children undergoing surgery required reoperation for recurrent subaortic stenosis at an average of 4.9 ± 0.9 years after initial resection. These patients initially presented with significantly higher gradients (66 ± 10 mm Hg).
Arguing against a more aggressive approach of surgical resection of membranous subaortic stenosis at the time of diagnosis, these data suggest that a significant proportion of these patients will have stable or at least slowly progressive gradients. Although the management approach remains variable, it seems reasonable to pursue surgical resection for peak systolic gradients of 40 mm Hg or greater (obtained by echocardiography). The new onset of aortic insufficiency should probably be considered an important indication for surgery, regardless of the gradient. Although Rohlicek and coworkers29 did not discern any improvement in aortic insufficiency after operation, it has been the experience of others that careful débridement of fibrous tissue encroaching on the aortic leaflets often results in significant improvement in aortic insufficiency.30
Results of Membranous Subaortic Stenosis
Membranous subaortic stenosis has been the subject of intense scrutiny, not only for its incidence but also for its propensity for recurrence after seemingly complete surgical resection. The reported recurrence rate for membranous subaortic stenosis has been reported to be between 0% and 55%, with most reports documenting a recurrence rate of 15% to 21%.28,31–35
Independent predictors of reoperation for recurrent subaortic stenosis include proximity of the membrane to the aortic valve (<6 mm) and a peak gradient of greater than 60 mm Hg. Adherence of the membrane to the aortic or mitral valve identified at surgery was also found to be a predictor for recurrence.36
Septal myotomy, in addition to membrane resection, has been reported to reduce the incidence of recurrence. Lupinetti and coworkers37 reported a recurrence rate of 4% when a septal myomectomy was performed in addition to membrane resection. This finding has not been confirmed by other studies, however.25,38
Other surgeons advocate an even more aggressive approach. Yacoub and colleagues31 suggested that an important pathologic feature of membranous subaortic stenosis is fibrous tissue ingrowth at the point of insertion of the anterior leaflet of the mitral valve into the left ventricular myocardium (Fig. 123-4). This ingrowth interferes with dynamic widening of the outflow tract, including posterior displacement of the mitral apparatus, during systole.
Adding resection of the fibrous tissue from the mitral valve trigones to membrane resection and septal myomectomy, Yacoub and colleagues31 reported excellent relief of LVOT gradient (mean, 8 mm Hg) in 57 patients. Over an average follow-up of 15 years, seven patients had mild to moderate aortic regurgitation. Most notably, no patient has required reoperation for recurrent stenosis.
Although periodic attempts at balloon dilation have been performed, the resulting gradients are generally high (about 30 mm Hg), and subsequent surgery is required in a majority. Among 13 patients undergoing dilation, Moskowitz and colleagues39 reported that nine required subsequent surgery. Suarez de Lezo and colleagues40,41 reported a 50% recurrence rate within 3 years. At this time, balloon dilation should not be considered a primary treatment of discrete subaortic stenosis.
Tunnel-Like Stenosis
Long-segment or tunnel-like stenosis has been defined as a muscular or fibromuscular subaortic stenosis, the length of which was more than one-third the aortic diameter.42 Although tunnel-like stenosis may be associated with other lesions (such as interrupted aortic arch and atrioventricular septal defect), this discussion will be confined to long-segment stenosis in the setting of concordant atrioventricular and ventriculoarterial connections with an intact ventricular septum.
Treatment
Modified Konno Operation
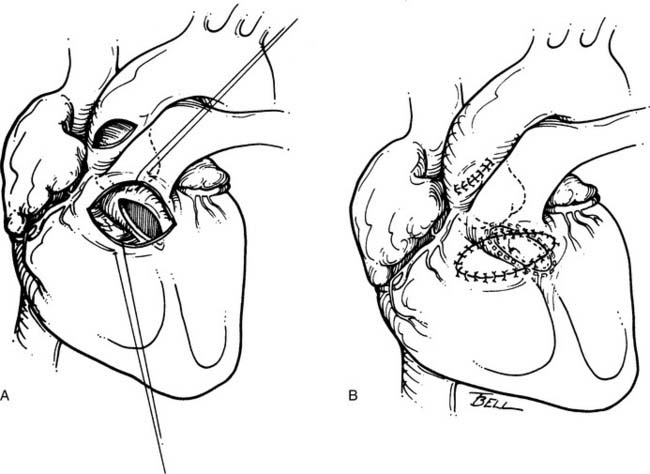
The degree of hypoplasia encountered in tunnel-like subaortic stenosis does not lend itself to transaortic resection, as might be performed for membranous subaortic stenosis. Surgery for tunnel-like stenosis is a more extensive procedure. In the presence of a suitable aortic valve, the modified Konno has become a favored operation for tunnel-like subaortic stenosis. It has the great appeal of enlarging the LVOT while preserving the native aortic valve. Excellent technical reviews of this technique are available.43,44
After inspection of the aortic valve, the infundibulum of the right ventricle is incised in a transverse fashion. Visualization of the ventricular septum from these two perspectives (through the aortic valve and through the infundibulum) allows an accurate incision through the ventricular septum (Fig. 123-5). Indentation of the safe area of ventricular septum (leftward of the nadir of the right coronary leaflet) with a right-angled clamp placed through the aortic valve allows an accurate incision through the septum via the infundibulotomy. It is important that the trajectory of this incision be directed toward the apex of the left ventricle, which, from the surgeon’s perspective, is almost directly leftward, and carried as distally as necessary to relieve the obstruction. Proximally, the incision may be carried up to, and if necessary into, the commissure between the right and left aortic leaflets. Septal tissue may be débrided from the left ventricle side of the septum, but this should be on the leftward portion of the incision, to spare the left-sided conduction apparatus as it courses down the ventricular septum. The resulting VSD is then closed with a generous Gore-Tex patch, augmenting the LVOT. The aortic valve is carefully débrided of any fibrous tissue, and the incisions are closed.
Indications for the Modified Konno Operation, and Results
The indications for the modified Konno operation for the relief of subaortic obstruction are evolving. Roughneen and coworkers45 described their experience with the operation in 16 children. Indications for operation were recurrent subaortic stenosis (n = 3), hypertrophic subaortic stenosis (n = 3), tunnel stenosis of the LVOT (n = 2), and subaortic stenosis after atrioventricular septal defect (n = 2), or VSD (n = 5). The modified Konno operation was very effective in reducing the LVOT gradient (mean, 50 to 3 mm Hg) . Caldarone and colleagues46 reported similar results for 18 patients. Although the technique is very effective in relieving subaortic stenosis resulting from a variety of lesions, complications can occur. In general, operative mortality in published reports has been negligible. However, right bundle branch block has been commonly noted after the modified Konno operation. This is significant in the setting of preexisting left bundle branch blocks, as may exist after transaortic resection of subaortic stenosis, and complete heart block has been noted in up to 12.5% of cases. Although the aortic valve is at risk, and residual VSDs may occur, these complications are uncommon with careful technique.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree