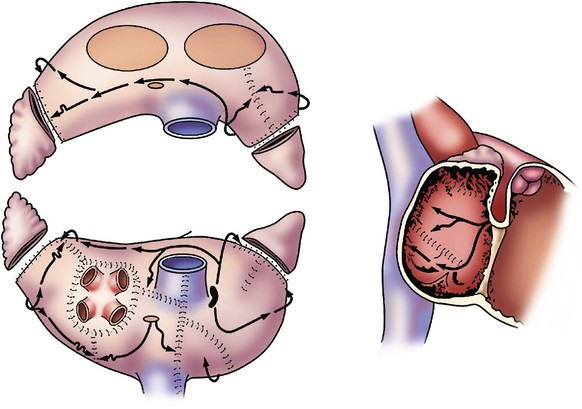
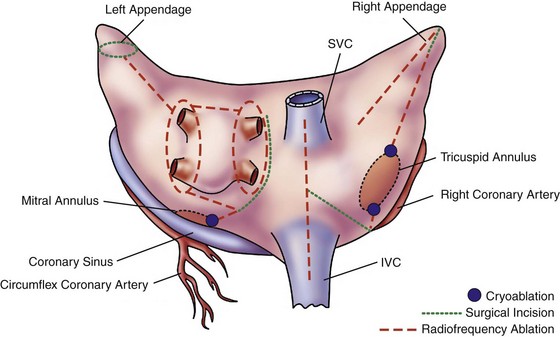
128 The need for interventional therapies to treat atrial fibrillation (AF) has arisen from the shortcomings of pharmacologic management. Antiarrhythmic medications have been limited by modest efficacy and significant proarrhythmic and systemic toxicities.1 Conversely, rate-control strategies leave the patient in AF, do not address the impaired hemodynamics or symptoms associated with this arrhythmia, and can render subsequent attempts at rhythm control therapies less effective when young patients suffer increased remodeling from spending prolonged periods of time in AF. Both rate and rhythm control strategies necessitate the use of coumadin for anticoagulation and as a result carry a defined risk of major bleeding. Theoretically, restoration of normal sinus rhythm has several potential benefits over other strategies. These benefits include improvements in atrial systolic function, which improves cardiac output and prevents the development of worsening symptoms in patients with congestive heart failure; lower risk of stroke; possible discontinuation of anticoagulation; and potential reverse atrial structural or electrical remodeling. Although the strategy of rhythm-control has never been shown to be superior to rate control, largely because of the aforementioned problems, a follow-up analysis of outcomes in the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial showed that the presence of normal sinus rhythm was associated with a significant 47% reduction in mortality.2 Given the significant prevalence and public health burden of AF, restoration of normal sinus rhythm with surgical ablation may be of significant benefit to selected patients. The first effective interventional procedure for AF was introduced clinically by Dr. James Cox in 1987 after extensive animal investigation at Washington University. This operation, the Cox-Maze procedure, was developed to provide a standardized anatomical approach that would be applicable to all patients. After initial attempts in the laboratory and the operating room failed to define the precise mechanisms of AF in the individual patient, this operation was designed to prevent AF by interrupting all potential macroreentrant circuits that could develop in the atria, thereby precluding the ability of the atrium to flutter or fibrillate (Figure 128-1). The operation consisted of myriad incisions arranged across the left and right atria in such a fashion that the sinoatrial node could still direct the propagation of the sinus impulse. The patterns of incisions were designed such that impulse propagation into dead end pathways would allow sufficient atrial myocardial depolarization to ensure contraction while preventing reentry. The Cox-Maze procedure was successful in restoring sinus rhythm and atrioventricular synchrony, thereby significantly reducing the risk of thromboembolism and hemodynamic compromise from the arrhythmia.3,4 The procedure also allowed for the preservation of atrial transport function in most patients.5 The Cox-Maze procedure has evolved through several iterations as clinical experience has increased and technology has advanced. The early versions of the Cox-Maze procedure were complicated by late chronotropic incompetence and a high incidence of postoperative pacemaker requirement. The Cox-Maze III, the third iteration, became the standard for the surgical treatment of AF.4 Despite its clinical success, it was not widely adopted because it remained technically demanding and significantly prolonged the need for cardiopulmonary bypass. During the last decade, many groups around the world have attempted to make the operation simpler and faster to perform by replacing the traditional cut-and-sew lesions with ablation lines created using various energy sources.6 In 2002, Gaynor et al.7 introduced the Cox-Maze IV operation, which uses a combination of bipolar radiofrequency ablation and cryoablation to effectively replace the majority of incisions that compose the Cox-Maze III (Figure 128-2). Figure 128-2 Diagram illustrating the incisions, lesions, and energy sources that are typically used for the Cox-Maze IV procedure. SVC, Superior vena cava; IVC, inferior vena cava. (From Weimar T, Bailey MS, Watanabe Y, et al: The Cox-Maze IV procedure for lone atrial fibrillation: a single center experience in 100 consecutive patients, J Interv Card Electrophysiol 31:47–54, 2011.) These ablation-based procedures have resulted in a dramatic increase in the number of operations performed annually for AF. In 2005, 12,737 patients were reported to the Society of Thoracic Surgeons Database as having undergone a surgical procedure for AF, whereas 1 year earlier the number was 3987 patients.8 Before 2004, the volume was so low that the operation was not reported. The development of surgical ablation technology has transformed the field of arrhythmia surgery by reshaping the Cox-Maze procedure into an operation that is technically easier, shorter, and less invasive. Several devices have been implemented with varying success; therefore, it is imperative that the relative advantages and disadvantages of each available modality are understood.6,9 Several criteria must be met for an ablation device to be considered suitable for arrhythmia surgery. First, it must reliably produce bidirectional conduction block across the line of ablation. It is important to understand that this block requires a transmural lesion, because even small gaps can conduct both sinus and fibrillatory wavefronts.10 Second, the ablation device must be safe; this requires a precise definition of dose-response curves to limit excessive or inadequate ablation and potential hazards to surrounding vital cardiac structures, such as the coronary sinus, coronary arteries, and valvular structures. Third, the ablation device should facilitate efficient completion of the operation. This requires that the device create lesions rapidly, be intuitive to use, and have adequate length and flexibility. Finally, the device should be adaptable to a minimally invasive approach. This adaptability would include the ability to insert the device through minimal-access incisions or ports. For the treatment of lone AF, devices that are able to create transmural lesions on the beating heart without the need for cardiopulmonary bypass are desirable. The failure to create linear epicardial lesions reliably on a beating heart is the biggest shortcoming of unipolar energy sources, and it has resulted in the development of hybrid procedures to allow for less invasive surgical options. Currently, no single device has properly met all these criteria. Cryoablation is unique in that it destroys myocardial tissue by freezing rather than heating. Because this technology preserves the myocardial fibrous skeleton and collagen structure, it is considered safe for use around valvular tissue. Lesion size and depth depend on the probe temperature, probe size, the duration and number of freeze cycles, gas type, and the thermal conductivity and temperature of the tissue.6 There are currently two commercially available sources of cryothermal energy that are being used in cardiac surgery: nitrous oxide and argon. At one atmosphere of pressure, nitrous oxide is capable of achieving a temperature of −89.5° C, whereas argon has a minimum temperature of −185.7° C. The nitrous oxide technology has been extensively used and is generally safe and efficacious, except around the coronary arteries where studies have shown late intimal hyperplasia after cryoablation.11 Disadvantages of cryoablation include the relatively long time required to create lesions (1 to 3 minutes) and the difficulty encountered when attempting epicardial creation of transmural lesions on the beating heart.6 The latter shortfall is in part a result of dissipation of thermal energy through convective warming from the circulating endocardial blood, which is known as the heat-sink effect. Finally, there is a risk of thromboembolism if blood freezes and coagulates during epicardial ablation on the beating heart. This problem can be overcome by placing the probe endocardially and freezing outward; however, this requires cardiopulmonary bypass. Radiofrequency (RF) was among the earliest energy sources to be applied reliably in the operating room for the treatment of AF. RF devices emit electromagnetic energy through the delivery of high-density, unmodulated alternating current at a frequency of 350 to 1000 kHz.6 This frequency is high enough to prevent rapid myocardial depolarization and induction of ventricular fibrillation yet low enough to prevent tissue vaporization and perforation. Approximately 1 mm of tissue adjacent to the probes is directly heated by a resistive effect, and deeper tissue layers are heated via conduction. Tissue temperatures of approximately 50 to 60° C result in coagulation and permanent destruction of cell structures and collagen. The lesion size depends on the electrode-tissue contact area, the interface temperature, the current and voltage (power), topical cooling, and tissue resistance. Accordingly, the depth of the lesion can be limited by char formation, epicardial fat, myocardial and endocardial blood flow, and tissue thickness. There have been a number of unipolar RF devices developed for ablation. Although unipolar RF devices have been shown to create transmural lesions on the arrested heart in animals with sufficiently long ablation times, they have not been consistently successful in humans. After 2-minute endocardial ablations during mitral valve surgery, only 20% of the in vivo lesions were transmural.12 Epicardial ablation on the beating heart has been even more problematic. Animal studies have shown consistently that epicardial application of unipolar RF is incapable of creating reliable transmural lesions on the beating heart,13 and epicardial RF ablation in humans resulted in only 10% of the lesions being transmural.14 Bipolar RF clamps were developed to overcome this problem by embedding the electrodes in the jaws of a clamp to focus the delivery of energy. This clamp shields the electrodes from the circulating blood pool and surrounding tissues, which shortens lesion formation and limits collateral injury. Bipolar ablation has been shown to be capable of creating precise transmural lesions on the beating heart both in animals and humans, with ablation times typically between 10 and 20 seconds.6,15 Bipolar devices have also been shown to be safer than unipolar devices. Although relatively infrequent, a number of clinical complications from unipolar RF devices have been reported, including coronary artery injuries, cerebrovascular accidents, and esophageal perforation leading to atrioesophageal fistula.6 Bipolar RF technology has virtually eliminated this collateral damage by confining the energy within the jaws of the clamp, and no device-related injuries have been reported in the literature with the use of bipolar RF clamps. Currently available devices use algorithms capable of predicting lesion transmurality either by measuring the tissue conductance between electrodes or through the use of temperature-based feedback. As a result, these devices can tailor the energy delivery to the physiological characteristics of tissue. High-intensity focused ultrasound (HIFU) is another modality applied clinically for surgical ablation. With these devices, ultrasound waves travel through the tissue causing compression, refraction, and harmonic oscillation in the carrier particles (i.e., water), which are translated into kinetic energy, ultimately creating thermal coagulative tissue necrosis. HIFU is the one unipolar source that produces high-concentration energy in a focused area at a defined distance from the probe. It is reportedly able to create transmural epicardial lesions through epicardial fat in less than 2 seconds without affecting intervening and surrounding tissue.6 There is a steep temperature gradient between the focused energy and collateral tissue with the targeted tissue rapidly raised to 80° C. A few clinical studies using HIFU have shown encouraging results16; however, more recent clinical experience has shown low efficacy and serious complications associated with minimally invasive use.17,18 Moreover, there has been no independent experimental verification of the efficacy of HIFU devices to reliably create transmural lesions, and the fixed depth of penetration of these devices can be problematic because of the variability of atrial wall thickness in pathological states. These devices are also somewhat bulky and expensive to manufacture. Because ultrasound is absorbed approximately thirtyfold more strongly in soft tissue than in low-viscosity liquid, the acoustic energy supplied by HIFU provides significantly more thermal damage to tissue than blood. The result is that the high blood flow through the coronary arteries provides a protective cooling of the endothelial lining, allowing the tissue surrounding the coronary arteries to be ablated without damaging the arteries themselves.16 This technology has not been widely used clinically, but several cases of serious complications, such as atrioesophageal fistula, phrenic nerve palsy, and esophageal perforation, have been documented.19 The Cox-Maze procedure is indicated for patients with medically refractory, lone AF and for patients undergoing cardiac surgery who have concomitant AF that would benefit from treatment. The latter group is sizeable and represents the majority of patients in most surgical clinical experiences. In a review of cases at Washington University from 1996 to 2005, the incidence of preoperative AF was 22% and 24% in patients referred for valvular or combined valvular/coronary surgery, respectively. The role of surgery for AF has been recently clarified and endorsed in a consensus statement released by The Heart Rhythm Society in partnership with the European Heart Rhythm Association, the European Cardiac Arrhythmia Society, the American College of Cardiology, the American Heart Association and the Society of Thoracic Surgeons.20 Surgical ablation for atrial fibrillation is indicated for: (1) all symptomatic AF patients undergoing other cardiac surgery; (2) selected asymptomatic AF patients undergoing cardiac surgery in which the ablation can be performed with minimal additional risk; and (3) patients with symptomatic lone AF who prefer a surgical approach, have failed one or more attempts at catheter ablation, or are not candidates for catheter ablation. Thus, for lone AF, surgery is a complimentary approach to catheter ablation. A relative indication for surgery exists for patients with AF who are at high risk for cerebrovascular thromboembolic events. This would include patients with persistent AF and a CHADS2 score of 2 or greater and who develop a contraindication to long-term anticoagulation.21 The annual risk of stroke in patients with a CHA2DS2-VASc score greater than 2 is between 3% and 15%,22 whereas the annual risk of stroke after a Cox-Maze procedure in 433 patients followed for a mean of 6.6 ± 5 years was 0.2%. In the latter study, there was no association between postoperative neurologic events and either the CHADS2 score or warfarin use.21 Although these benefits have not been demonstrated in prospective, randomized trials, they are thought to be a result of both successful restoration of sinus rhythm and exclusion of the left atrial appendage, which is considered a major source of thromboemboli in patients with AF. Surgical treatment for AF with amputation of the left atrial appendage should also be considered in high-risk patients with both persistent AF and previous cerebrovascular events that occurred while receiving therapeutic anticoagulation, because these patients are at high risk for further embolic events. Coumadin is capable of reducing the risk of ischemic and hemorrhagic strokes by more than 60% in patients with AF, but it does not completely eliminate this serious complication.23 In the study, 20% of patients who underwent the Cox-Maze III procedure had experienced at least one episode of significant cerebral thromboembolism preoperatively.4 Furthermore, studies have shown that adding the Cox-Maze procedure in patients undergoing concomitant valve surgery can decrease the late risk of cardiac- and stroke-related deaths.24 A series from Japan demonstrated a 10% increase in incidence of stroke at 8-year follow-up for patients with chronic AF who underwent mitral valve replacement alone compared with similar patients who had mitral valve replacement with a concomitant Cox-Maze procedure.25 The Cox-Maze III is rarely performed, because most centers have replaced the surgical incisions described in the original cut-and-sew procedure with lines of ablation created by a variety of different energy sources. Bipolar RF energy and cryoablation have been used successfully to replace most of the surgical incisions of the Cox-Maze III procedure in an operation termed the Cox-Maze IV (see Figure 128-2). The Cox-Maze IV procedure is performed on cardiopulmonary bypass using either a median sternotomy, often in combination with other cardiac surgery, or a less-invasive right minithoracotomy. All patients undergo intraoperative transesophageal echocardiography, and if a patient is in AF at the time of surgery, amiodarone is administered and the patient is electrically cardioverted after excluding the presence of a left atrial clot. Both the right and left pulmonary veins (PVs) are bluntly dissected. Pacing thresholds are measured from each PV. The PVs are then isolated using a bipolar RF ablation device, such that a linear line of ablation surrounds a cuff of atrial tissue encompassing the right and left PVs, respectively (Figure 128-3
Surgery for Atrial Fibrillation and Other SVTs
Atrial Fibrillation
Development of Surgery for Atrial Fibrillation
Surgical Ablation Technology
Cryoablation
Radiofrequency Energy
High-Intensity Focused Ultrasound
Indications for Surgical Ablation of Atrial Fibrillation
The Cox-Maze Procedure
Surgical Technique
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Surgery for Atrial Fibrillation and Other SVTs