Surgery for Achalasia and Other Motility Disorders
Farzaneh Banki
Tom R. DeMeester
The diagnosis and treatment of esophageal motility disorders can be an important part of a thoracic surgeon’s practice. The majority of patients with esophageal motility disorders are referred to surgeons following a primary assessment by a gastroenterologist. The patients are commonly on various medications and have undergone multiple endoscopies and dilatations. The effects of these treatments may be of some benefit but the patient’s symptoms remain and a more definitive solution is desired. It is crucial for the thoracic surgeons prior to surgery to validate the accuracy of the patient’s diagnosis. To do so, they must understand esophageal physiology, be able to perform a thorough esophageal assessment, and determine the best treatment option.
Since the last edition of this book, there has been a shift in the surgical approach to esophageal motility disorders. A left thoracotomy, once the standard approach to perform a Heller myotomy, has been replaced by the laparoscopic approach. Similarly, sophisticated diagnostic tools have been developed, such as high-resolution manometry, esophageal impedance, and 48-hour Bravo pH capsule. In this chapter, we will review the diagnosis and treatment of the primary and secondary esophageal motility disorders. We will focus on the diagnostic assessment and the use of endoscopic, minimally invasive, and open surgical therapy.
CLASSIFICATION OF ESOPHAGEAL MOTILITY DISORDERS
Primary esophageal motility disorders (absence of extra esophageal causes)
Achalasia
Diffuse esophageal spasm
Nutcracker esophagus
Hypertensive lower esophageal sphincter
Ineffective esophageal motility
Secondary esophageal motility disorders (due to extra esophageal causes)
Collagen vascular disease (scleroderma, polymyositis, dermatomyositis, systemic lupus)
Neuromuscular disease (myasthenia gravis)
Endocrine and metabolic disorders
ACHALASIA
Definition, Prevalence, and Natural History
Achalasia is a primary esophageal motility disorder that can affect the esophageal body, lower esophageal sphincter, and stomach. It is characterized by the absence of peristaltic contractions in the esophageal body, a hypertensive lower esophageal sphincter, complete or nearly complete loss of the lower esophageal sphincter relaxation on swallowing and, an elevation of esophageal luminal pressure above gastric baseline pressure. Some patients may also have delayed gastric emptying. The latter can occur years after the onset of the disease.
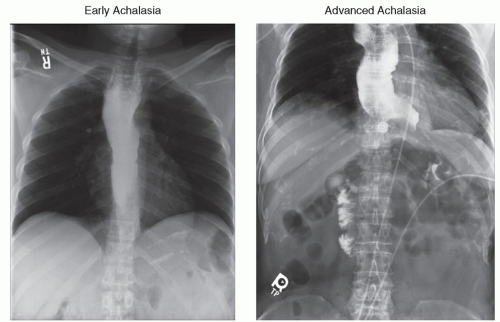
The frequency of the disease in the western world is one per 100,000 population per year with geographic pockets of higher incidences. There are two peaks in the occurrence of the disease, one between ages 20 and 40 and the other between 60 and 70. If left untreated, achalasia will cause a dilated esophagus from the accumulation of liquid within the lumen. The weight of the retained liquid column causes the loss of the normal esophageal axis and the formation of a sigmoid esophagus. Eventually, the patient loses the ability to drink water or take solid nourishment. In the past, this resulted in the death of the patient from dehydration and starvation. Patients with achalasia have higher risk for squamous cell carcinoma of the esophagus.
Vigorous Achalasia
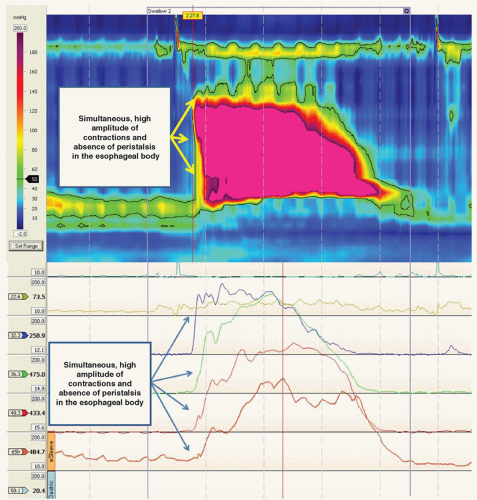
A subgroup of patients with achalasia have isolated simultaneous segmental contractions in the esophageal body of high amplitude (>180 mmHg). These patients present with chest pain in addition to dysphagia. These patients do not do as well after surgical therapy due to the persistence of chest pain in about 50% of those operated on.
Etiology and Symptoms
The cause of achalasia is unknown. Hereditary, degenerative, autoimmune, and viral infections have been identified as possible etiologies. The most common symptom is dysphagia, which is reported in 94% of patients, followed by regurgitation in 76% and chest pain in 41%.
Up to half of the patients may complain of heartburn from esophageal distension, acidic fermentation of retained food, or delayed clearance of rare reflux episodes.
Diagnosis
A videoesophagram, an esophageal motility study, and an upper endoscopy should be obtained in patients suspected of having achalasia. Computed tomography (CT) scan and pH monitoring are selectively used.
Videoesophagram
In patients with early achalasia, the esophagus is normal in diameter and peristalsis is absent. The distal esophagus is characterized by a smooth tapering of the barium column resembling a bird’s beak. As the disease progresses, the esophagus becomes more dilated and tortuous. Retained food and saliva collect within the esophageal lumen and form an air-fluid level when the patient is in the upright position (Fig. 20.1). A gastric air bubble is typically absent.
Upper endoscopy
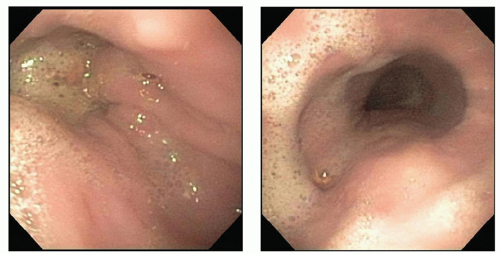
In patients with achalasia, upper gastrointestinal endoscopy shows a dilated
esophagus containing foamy saliva and retained food (Fig. 20.2). The gastroesophageal junction is closed but allows a 9-mm endoscope to be pushed through the junction with minimal but definite resistance. Rarely is balloon dilation required to facilitate the passage of the scope. Pseudoachalasia can occur secondary to esophageal obstruction from a malignant lesion at the level of the gastroesophageal junction. Upper endoscopy usually can exclude this possibility, but occasionally the diagnosis can be difficult due to normal mucosa that overlies a submucosal tumor. A CT scan usually resolves the issue. The upper gastrointestinal endoscopy should be performed by the operating surgeon prior to the day of the surgery. If performed immediately prior to surgery, the upper abdominal viscera can become distended and obstruct the laparoscopic image.
esophagus containing foamy saliva and retained food (Fig. 20.2). The gastroesophageal junction is closed but allows a 9-mm endoscope to be pushed through the junction with minimal but definite resistance. Rarely is balloon dilation required to facilitate the passage of the scope. Pseudoachalasia can occur secondary to esophageal obstruction from a malignant lesion at the level of the gastroesophageal junction. Upper endoscopy usually can exclude this possibility, but occasionally the diagnosis can be difficult due to normal mucosa that overlies a submucosal tumor. A CT scan usually resolves the issue. The upper gastrointestinal endoscopy should be performed by the operating surgeon prior to the day of the surgery. If performed immediately prior to surgery, the upper abdominal viscera can become distended and obstruct the laparoscopic image.
Esophageal Manometry
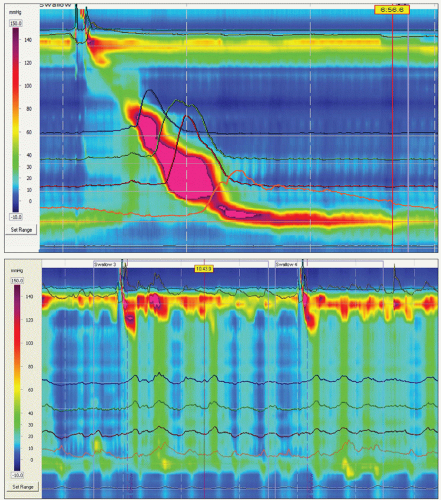
The gold standard for the diagnosis of achalasia is the complete or nearly complete loss of the lower esophageal sphincter relaxation in response to wet swallows and the absence of peristalsis in the esophageal body on esophageal manometry (Fig. 20.3). A hypertensive lower esophageal sphincter is present in less than half of the patients. Commonly, there is an elevated esophageal baseline luminal pressure reflecting outflow resistance. Patients with vigorous achalasia have in addition isolated segmental contractions of high amplitude (>180 mmHg; Fig. 20.4).
Computed Tomography Scan
A CT scan is not necessary except when pseudoachalasia is suspected, and the gastroesophageal junction needs to be imaged to identify a mass compressing the esophagus.
pH Monitoring
pH monitoring is performed when the diagnosis of achalasia is questionable and gastroesophageal reflux disease is suspected. It is not uncommon to confuse the two entities. Achalasia patients can complain of heartburn from esophageal distension, acidic fermentation of retained food, or delayed clearance of rare reflux episodes. To exclude gastroesophageal reflux disease, pH monitoring should be performed. The pH tracing must be carefully analyzed to distinguish the episode of gastroesophageal reflux from long periods of low pH secondary to fermentation of retained food or episodes of rare reflux that clears slowly from the esophagus.
Treatment
The goal of therapy for achalasia is deceptively simple: to relieve dysphagia by reducing esophageal outflow resistance without causing unimpeded reflux of the gastric juice. To achieve this goal is more difficult than it sounds.
Nonsurgical Treatment Botox Injection
Botulinum toxin inhibits the calciumdependent release of acetylcholine from nerve endings involved in the relaxation of the lower esophageal sphincter. It is therapeutically effective in relieving dysphagia in about 85% of patients. Over a period of 6 months, the symptoms recur in more than 50% of the patients possibly because of regeneration of the affected receptors. Patients older than 60 are likely to have a sustained response lasting for 1 to 5 years.
A Heller myotomy after Botox injection can be difficult and less effective
because of submucosal scar formation. The fibrotic reaction can obliterate the anatomical plane between the mucosa and the muscular layer of the esophagus. This increases the risk of mucosal perforation when performing the myotomy. For this reason, Botox injections are used only in high-risk elderly patients with significant comorbidities.
because of submucosal scar formation. The fibrotic reaction can obliterate the anatomical plane between the mucosa and the muscular layer of the esophagus. This increases the risk of mucosal perforation when performing the myotomy. For this reason, Botox injections are used only in high-risk elderly patients with significant comorbidities.
Pneumatic Balloon Dilation
Pneumatic balloon dilation of the lower esophageal sphincter is the most effective nonsurgical treatment for achalasia. A special balloon, used to perform dilatation, is inflated to a diameter of 30 to 40 mm or a circumference of 90F to 120F. The procedure is monitored under fluoroscopy, and the balloon is commonly filled with liquid contrast for a better visualization during inflation. The waist of the balloon is positioned at the level of the hypertrophied lower esophageal sphincter and is inflated to a diameter of 30 mm. A follow-up procedure can be performed if dysphagia persists. This is usually done by inflating the balloon to a diameter of 40 mm. The procedure is associated with a 1.6% to 4% risk of esophageal perforation. Predictors of a poor outcome are an age <40 and a lower esophageal sphincter pressure >10 mmHg 3 months after the procedure.
Surgical Treatment
Heller Myotomy
The most effective and durable treatment for achalasia is a Heller myotomy. Initially, this procedure was performed transthoracically either via a thoracotomy or
thoracoscopy. Today, the procedure is universally performed by laparoscopy. Critical steps of the operation as done by us involve performing a left lateral myotomy in line with the greater curvature of the stomach, starting at the apex of the diaphragmatic esophageal hiatus dividing the esophageal muscle, all the oblique fibers at the angle of His and extending the myotomy 3 cm beyond the gastroesophageal junction onto the stomach. The loss of esophageal axis and a long duration of symptoms are predictors of a poor outcome.
thoracoscopy. Today, the procedure is universally performed by laparoscopy. Critical steps of the operation as done by us involve performing a left lateral myotomy in line with the greater curvature of the stomach, starting at the apex of the diaphragmatic esophageal hiatus dividing the esophageal muscle, all the oblique fibers at the angle of His and extending the myotomy 3 cm beyond the gastroesophageal junction onto the stomach. The loss of esophageal axis and a long duration of symptoms are predictors of a poor outcome.
Fundoplication after Heller Myotomy
After Heller myotomy, 40% to 60% of patients have gastroesophageal reflux. For this reason, it is recommended that a partial fundoplication should be added. A prospective randomized trial of myotomy alone or myotomy plus Dor fundoplication showed a reduction in postoperative reflux from 48% to 9%, respectively. In contrast, a more recent randomized trial on the degree of partial fundoplication, that is a 180-degree Dor anterior fundoplication versus a 270-degree Toupet posterior fundoplication, did not show a difference in reflux protection. The advantage of a Toupet fundoplication is the retraction of the edges of the myotomy, potentially reducing reapproximation and healing of the myotomized muscle edges together resulting in recurrent dysphagia. The disadvantages are the need to dissect posterior to the esophagus with the potential to damage the posterior vagus nerve and leaving the mucosa at the myotomy site uncovered. In contrast, the Dor fundoplication does not require posterior dissection and covers the mucosa at the myotomy site.
Surgical Technique of Heller Myotomy and Dor Fundoplication
The patient is placed on the operating table and positioned in the reverse Trendelenburg with the lower extremities spread apart in leg holders. A 1.5-cm incision is made just to the left of the midline, two-third the distance between the xiphoid and the umbilicus. An 11-mm Opti-port and a zero-degree scope are used to obtain access into the abdominal cavity under direct vision. The zero-degree scope is exchanged for a 30 degree scope. Under direct vision, a 12-mm multipurpose port is placed in the left upper quadrant, a 5-mm retraction port in the left lower quadrant, a 5-mm dissection port in the right upper quadrant, and a 5-mm port is used to create a channel into the abdomen just below the xiphoid for the insertion of the liver retractor (Fig. 20.5). The liver retractor supplied with the “Iron Intern” is passed through the channel, placed under the left lobe of the liver, elevated and retracted to the right to expose the esophageal diaphragmatic hiatus (Fig. 20.6).
The superior upper third of the gastric greater curvature is mobilized by dividing the short gastric vessels. The posterior fundus is dissected off the lateral surface of the left crus. The left lateral wall of the esophagus is separated from the left crus and the esophagus is mobilized from the crural decussation up to the apex of the diaphragmatic hiatus and into the inferior mediastinum.
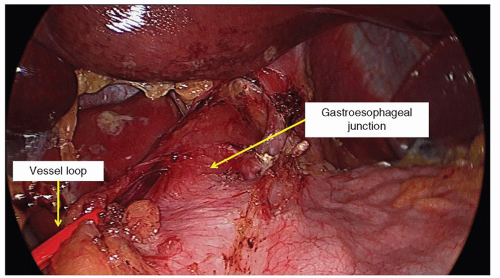
The gastroesophageal fat pad is dissected off the esophagus in a left-to-right direction allowing the right side of the fat pad to remain attached. A right-angle instrument is passed through the right upper quadrant dissection port and a small window is created in the base of the mobilized gastroesophageal fat pad at the point where the stomach flares from the tubular esophagus. A vessel loop is passed through the right upper quadrant dissection port, guided through the window and is brought back out through the right upper quadrant port site. The port is removed to free the vessel loop from the lumen of the port and the port is reinserted adjacent to the vessel loop. The esophagus is rotated to the right by retraction of the vessel loop so that the greater curvature of the stomach is in an anterior position (Fig. 20.7) of the stomach.
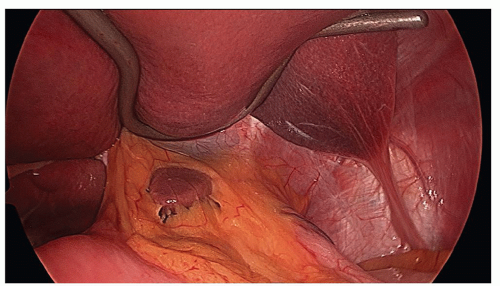 Fig. 20.6. The liver retractor is placed under the left lobe of the liver, elevated and is retracted to the right to expose the esophageal diaphragmatic hiatus. |
The myotomy is performed on the left lateral surface of the esophagus in line with
the greater curvature of the stomach. It extends from the apex of the hiatus down to the gastroesophageal junction and along the greater curvature of the stomach for 3 cm. Electrocautery is used to mark the site of proposed myotomy (Fig. 20.8). The myotomy is started by using a laparoscopic curved dissector to gently separate the longitudinal and circular muscular layers and identify the mucosa. This part of the operation must be meticulously performed. Once all the muscular fibers are separated, the mucosa bulges out. From this point, the myotomy is performed superiorly and inferiorly. In the superior direction, the esophageal muscle is divided by electrocautery using scissors up to the apex of the diaphragmatic hiatus. In the inferior direction, a laparoscopic hook is used to extend the myotomy down to the gastroesophageal junction and for 3 cm along the greater curvature of the stomach. The esophageal muscle is usually thick and white in appearance. Any remaining small septae on the mucosal surface are divided (Fig. 20.9). A Kittner dissector is used to push back the edges of the myotomized esophageal muscle to widen the myotomy and create a lip of the myotomized muscle at the edge of the myotomy. If persistent bleeding occurs from the muscle edges, it can be stopped with electrocautery; In doing so, care should be taken not to cauterize the mucosal surface.
the greater curvature of the stomach. It extends from the apex of the hiatus down to the gastroesophageal junction and along the greater curvature of the stomach for 3 cm. Electrocautery is used to mark the site of proposed myotomy (Fig. 20.8). The myotomy is started by using a laparoscopic curved dissector to gently separate the longitudinal and circular muscular layers and identify the mucosa. This part of the operation must be meticulously performed. Once all the muscular fibers are separated, the mucosa bulges out. From this point, the myotomy is performed superiorly and inferiorly. In the superior direction, the esophageal muscle is divided by electrocautery using scissors up to the apex of the diaphragmatic hiatus. In the inferior direction, a laparoscopic hook is used to extend the myotomy down to the gastroesophageal junction and for 3 cm along the greater curvature of the stomach. The esophageal muscle is usually thick and white in appearance. Any remaining small septae on the mucosal surface are divided (Fig. 20.9). A Kittner dissector is used to push back the edges of the myotomized esophageal muscle to widen the myotomy and create a lip of the myotomized muscle at the edge of the myotomy. If persistent bleeding occurs from the muscle edges, it can be stopped with electrocautery; In doing so, care should be taken not to cauterize the mucosal surface.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree