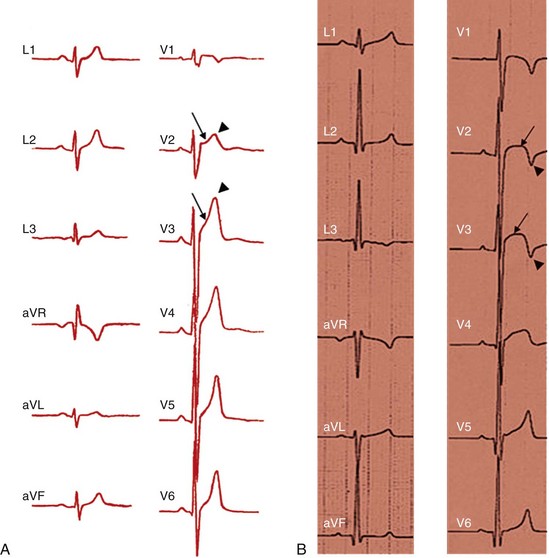
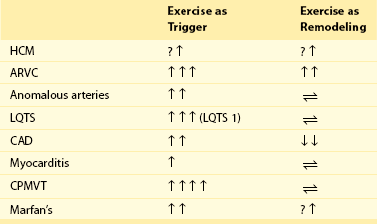
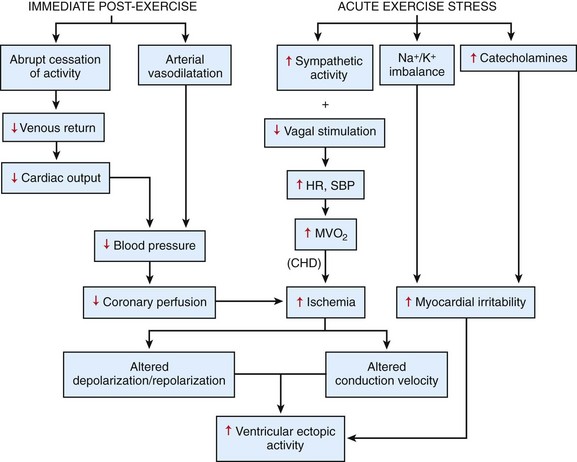
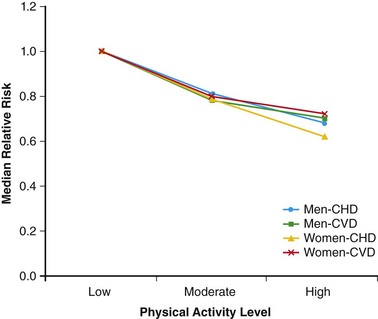
108 In the United States, approximately 150 young athletes die suddenly during sporting activities, for an incidence of 0.5/100 000 athletes a year.1,2 Similar incidences are seen in Italy.3 Based on the often intense media attention generated by SCD in the athlete, it appears that SCD deaths in athletes are much more common than in nonathletes. However, this impression is not borne out by the data. Early data from Italy show that the risk of SCD in athletes (2.3 in 100 000 per year) is greater than that in nonathletes (0.9 in 100 000 per year).4 In the United States, basketball players may be at higher risk of SCD, with one study reporting an incidence of 3.6 per 100 000 person-years in male basketball players,2 and in a more recent survey in National Collegiate Athletic Association (NCAA) colleges, the risk of SCD in male Division I basketball players was as high as 1 in 3100 person-years.5 However, most data do not support an increased risk of SCD in athletes.2,3,6–11 Data from the Padua region, which initially showed a higher risk of SCD in athletes, later demonstrated that death rates in athletes were similar to those in nonathletes.3 In most series of SCD in the young, deaths outside of sports far exceed deaths in sports. In the Resuscitation Outcomes Consortium (ROC), the incidence of sudden cardiac arrest in all children was 3.73 in 100 000 person-years between 1 and 11 years of age and 6.37 per 100 000 person-years between 12 and 19 years of age.11 Among a U.S. pediatric population of more than 74 million between 1 and 18 years of age (U.S. Census data, 2010: http://www.census.gov/prod/cen2010/briefs/c2010br-03.pdf), between 3000 and 5000 sudden cardiac arrests are reported per year. Reasonably good data indicate that 100 to 150 SCDs occur in sports.1 Thus, it appears that most SCDs in the young occur outside of athletics. This lower incidence of SCD in sports has also been seen in Germany and Denmark. Only 176 of 48 335 fatalities in Hamburg, Germany, were seen in sports.12 In Denmark only 15 of 5662 deaths in individuals between 12 and 35 years of age were sports related.8 The incidence of SCD in athletes (1.21/100 000 person-years) was lower than that in the general population (3.76/100 000 person-years). In a general pediatric population in Japan, the incidence of SCD was 1.32 per 100 000 person-years.9 Among U.S. military recruits, the incidence of SCD was 1.32 per 100 000 person-years, with a sudden death rate of 13 per 100 000 person-years.10 In a Dutch study, the incidence of cardiac arrest in the population <21 years old was 3.2/100 000 person-years.13 Thus, most data indicate a similar or lower incidence of SCD in athletes and nonathletes. Yet these data are far from complete because most athletic deaths are counted as athletic deaths only if they occur on the playing field. Deaths in athletes that occur outside of sports generally are not noted by the media and thus are not viewed as athletic deaths. As in nonathletes, most athletes with SCD have an underlying cardiac abnormality (Figure 108-1). In the United States, the most common underlying cardiac condition is hypertrophic cardiomyopathy (HCM).1 Coronary artery anomalies, myocarditis, Marfan’s syndrome, arrhythmogenic right ventricular cardiomyopathy (ARVC), valvular disease, and dilated cardiomyopathies account for most of the remaining cases of underlying heart disease. Commotio cordis, one of the few causes of SCD with a normal heart, accounts for nearly 20% of cases of SCD in athletes—the second most common cause in the United States.1 In Italian series of SCD in athletes, ARVC is found in 22%, and HCM is present in 2%.3 These changes in origin could be related to the Italian screening process for athletes but also could reflect different genetic penetrations of ARVC and HCM. Anomalous coronary arteries and coronary artery disease (CAD) account for a significant percentage of cases of SCD. Figure 108-1 Relative Incidence of Underlying Disease in SCD of Athletes in United States1 and Italy4 and of Military Personnel <35 Years of Age in United States22 and New Zealand21 Recently, in the general population, early repolarization has been reported to be associated with SCD.14,15 These reports are of potential concern for the athletic community because early repolarization is seen in up to 76% of athletes.16,17 However, it is apparent that in studies that report an association with SCD, early repolarization is defined differently than it had been defined previously.18 In studies linking early repolarization to SCD, abnormalities were defined as terminal slurring of the QRS or early notching of the J wave, unlike previous definitions, which primarily consisted of J point elevation with accompanying ST elevation (Figure 108-2).18 Early repolarization of the latter definition is very common among trained athletes; however, no data have suggested that early repolarization is a cause of SCD in athletes.19 And in fact, available data argue that the early repolarization pattern observed in the athlete is not a marker of increased arrhythmic risk.20 Figure 108-2 An ECG of the type of early repolarization described in athletes. J point elevation with ST elevation is seen in up to 76% of athletes.16,17 In nonathletic populations such as the military and the general public, causes of sudden death in the young differ from those of athletic SCD. Genetic cardiomyopathies are much less common, and myocarditis, anomalous coronary arteries, CAD, and normal hearts are more frequent.10,21,22 In nonpediatric populations, underlying CAD is often found. Sound data have linked acute triggering of SCD to athletic activity. In large, well-studied populations, vigorous activity temporarily increases the risk of SCD for the period during vigorous exertion and for 30 to 60 minutes afterward.23–26 In the Physicians Health Study, acute exercise temporally increased the risk of SCD 11-fold among individuals who were habitual exercisers and 74-fold in those who did not regularly exercise.24 Yet, even with the association of SCD with exertion, habitual exercise lowered all-cause mortality, likely by affecting the risk factors for CAD. How exercise triggers SCD is multifactorial and is related to hemodynamic and adrenergic stimulation (Figure 108-3).26 Triggering of SCD with exercise is best documented but still incompletely understood in the long QT (LQT) syndrome (Table 108-1).27,28 In LQT1, the most common trigger of syncope and SCD involves high sympathetic states such as exercise or fright. LQT1 patients, in particular, have SCD during swimming. However, individuals with LQT2 and LQT3 are not at increased risk of cardiac events with exercise, but rather with sleep. β-Blocking agents, which block the sympathetic surge with exercise, reduce the risk of arrhythmia in LQT1 but do not decrease the risk in LQT3.27,28 Table 108-1 Exercise as a Time-Specific Trigger to Arrhythmias and Sudden Cardiac Death and Exercise as a Potential Adverse or Beneficial Remodeler to Arrhythmias and Sudden Cardiac Death Figure 108-3 Exertion- and post-exertion–related physiologic changes that increase the risk of arrhythmias. (From Thompson PD, Franklin BA, Balady GJ, et al: Exercise and acute cardiovascular events placing the risks into perspective: A scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation 115:2358-2368, 2007; reproduced with permission.) Patients with catecholaminergic polymorphic ventricular tachycardia (CPVT) also are at increased risk of arrhythmias with exertion.29 Arrhythmias are typically provoked at the same level of exertion, and SCD during exertion is typical. β-Blockade is particularly effective in this condition. In patients with ARVC, arrhythmias may be provoked by exercise, and β-blockade may be effective in decreasing arrhythmias. Exercise-induced triggering of sudden death is not as well documented in HCM. Although some data suggest that competitive athletes with HCM are at increased risk for SCD, it is not clear whether this increased risk is due to increased triggering of arrhythmias or to remodeling of the substrate, which increases the risk of SCD. Epidemiologic data demonstrate that sudden cardiac death in athletes with HCM occurs most commonly in the afternoon—a time when training and competitive events are held.30 Many case reports and descriptions of athletes document SCD during exercise. Whether sympathetic drive affects the risk of commotio cordis is not entirely clear. In the Commotio Cordis Registry, nearly 60% of events occur during competitive sports. It is extremely unlikely that competitive sports account for 60% of the incidences of being struck in the chest by balls or other objects. Thus, it is possible that the high adrenergic drive associated with competition in commotio cordis also increases the risk of SCD.31 For the Brugada syndrome, myocarditis, and dilated cardiomyopathies, none of the current evidence indicates that exertion triggers arrhythmias.28 Epidemiologic data show the benefits of regular exercise in the prevention of CAD and the reduction of mortality (Figure 108-4).23,24,32 Reduction in cardiovascular events is likely brought about by a number of beneficial effects of exercise, including decreased weight and blood pressure, improved lipids and glucose tolerance, and increased adherence to a healthy lifestyle, which includes increased attention to diet and reduction in tobacco product consumption. This reduced risk of cardiac events is seen with chronic energy expenditure as minimal as walking and appears to increase with the both the time and the intensity of exercise.32,33 In general, the more vigorous the exercise, the greater are the long-term benefits. It has been argued that low cardiovascular fitness constitutes the largest attributable risk for all-cause mortality.34,35 American Heart Association physical activity guidelines call for 30 minutes of moderate intensity aerobic activity 5 days a week or vigorous intensity aerobic activity 3 days a week.36 In addition to reducing physical disease, evidence shows that sport participation, including but not restricted to competitive sports, reduces the risk of suicide—an especially important consideration for the young, among whom suicide is the third leading cause of death.37,38 Figure 108-4 Decrease in mortality associated with low, moderate, or high chronic physical activity. A dose response of benefit is noted, with more intense exercise providing the greatest benefit. (From Shiroma EJ, Lee IM: Physical activity and cardiovascular health: Lessons learned from epidemiological studies across age, gender, and race/ethnicity. Circulation 122:743-752, 2010; reproduced with permission.) However, for individuals with congenital heart disease, there has never been any documentation of reduced mortality with exercise.39 It may even be that the opposite is the case; habitual exercise and especially competitive exercise may increase the risk of sudden cardiac death.4 This potentially harmful effect is suggested by epidemiologic studies in which diseases such as HCM and ARVD are overrepresented in athletic populations with SCD compared with the general population. Mice data provide support for worsening of the ARVC phenotype with exercise.40,41 Some evidence suggests that overtraining may have adverse cardiovascular effects.42 Data from Italy show that intense training increases premature ventricular arrhythmias, but this increase appears to be benign.43 Other data for ventricular proarrhythmias in humans are anecdotal; these have been observed predominantly in a rat model of long-term intensive exercise.44 However, accumulating evidence shows a higher incidence of atrial fibrillation in athletes.45,46 It is believed that this increased risk is due at least in part to the high vagal tone of the athlete, and atrial fibrillation typically is initiated during sleep. With deconditioning, episodes of atrial fibrillation may be diminished. Increased left atrial dimensions seen in athletes47 and subsequent increases in atrial wall strain/stretch may play a role in the mechanism of atrial fibrillation.48 Abnormalities in ECGs of athletes are common (Table 108-2).49 Whether these alterations are due to conditioning effects of sports or to underlying cardiac pathology is of critical importance. In the Padua screening series, nearly 10% of the individuals screened had abnormal ECGs.50 Sinus bradycardias, first-degree heart block, and Mobitz type II Wenckebach are common, as are early repolarization and isolated voltage criteria for left ventricular hypertrophy.51 These minor abnormalities are unlikely to be associated with heart disease. However, it is clear that marked abnormalities, including repolarization abnormalities, pathologic Q waves, conduction defects, and ventricular arrhythmias, are far less common.52 Among another 32 652 Italian athletes, the prevalence of ECG abnormalities was 11.8%, including 2.3% with T wave inversion.53 Table 108-2 Classification of Abnormalities of the Athlete’s Electrocardiogram* LBBB, Left bundle branch block; RBBB, right bundle branch block. *Group 1 abnormalities are common and are not pathologic; group 2 changes are potentially signs of cardiac pathology. From Corrado D, Pelliccia A, Heidbuchel H, et al: Recommendations for interpretation of 12-lead electrocardiogram in the athlete. Eur Heart J 31:243-259, 2010, with permission. Abnormal ECGs in other populations are increased compared with the series in Italians (Table 108-3). In one U.S. series of professional football players, ECG abnormalities are seen in up to 55%, including ST abnormalities in up to 15% and intraventricular conduction defects (IVCDs) in up to 20%, with an increased prevalence of abnormalities in African American compared with Caucasian players.54,55 Another U.S. series of professional football players showed that black athletes were more likely than white athletes to have an abnormal ECG (30% vs. 13%; P < .0001).56 In a Dutch series of 428 athletes, 22% had T wave inversion or ST depression, and 7% had right or left bundle branch block (RBBB or LBBB).57 Among African athletes, the incidence of severe ECG abnormalities is markedly increased; up to 23% with T wave inversions and up to 13% with right ventricular hypertrophy are included.16,17 The reason for the increase in ECG abnormalities in other cohorts compared with Italian cohorts is not clear, but this increase clearly has implications for screening of athletes with ECGs. Adaptive myocardial remodeling is frequent among athletes. These reversible changes include left ventricular dilatation and hypertrophy. Left ventricular wall dimensions have been shown to increase to up to 14 mm in Italian and British athletes (Figure 108-5).58,59 Data show that American football players have even more marked myocardial hypertrophy of up to 16 mm.60 In a cohort of 156 asymptomatic National Football League individuals, 23% had evidence of left ventricular hypertrophy (LVH), including 6% with wall thicknesses >14 mm. A significant correlation has been noted between body weight and left ventricular wall thickness. In a series of British black athletes, the incidence of LVH was very similar to that in American footballers.61
Sudden Cardiac Deaths in Athletes, Including Commotio Cordis
Epidemiology of Sudden Cardiac Death in Athletes
Etiology of SCD in the Athlete
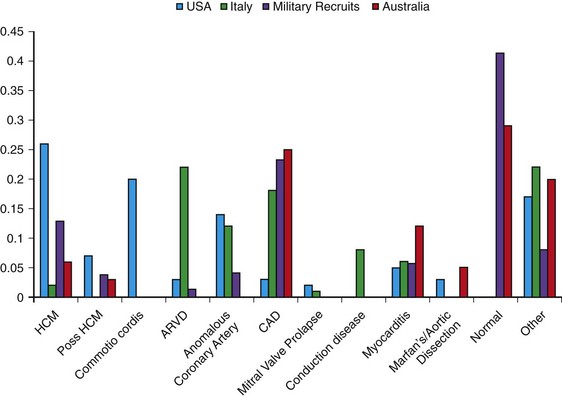
Note the regional differences in the incidences of HCM, commotio cordis, ARVC, coronary disease, and myocarditis. HCM is most commonly observed in U.S. athletes and ARVD in Italian athletes, but in nonathletic populations, anomalous coronary arteries, coronary artery disease, and myocarditis are more common causes of SCD.
Acute Triggering of SCD with Athletic Activity
Benefits of Exercise
Electrocardiograms in Athletes
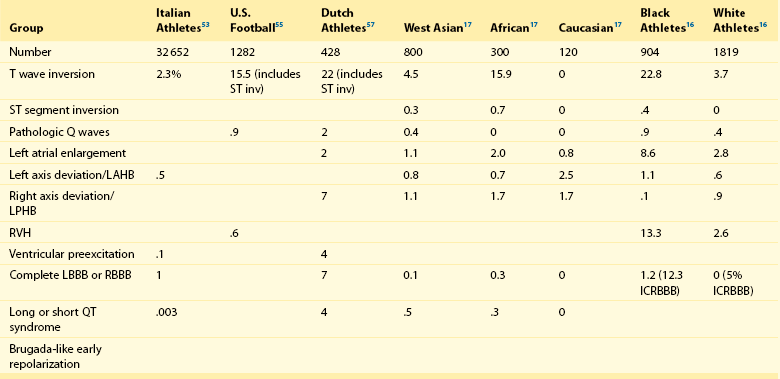
Group 1: Common and Training-Related ECG Changes
Group 2: Uncommon and Training-Unrelated ECG Changes
Sinus bradycardia
T wave inversion
First-degree AV block
ST segment inversion
Incomplete RBBB
Pathologic Q waves
Early repolarization
Left atrial enlargement
Isolated QRS voltage criteria
Left axis deviation/left anterior hemiblock
Right axis deviation/left posterior hemiblock
Right ventricular hypertrophy
Ventricular preexcitation
Complete LBBB or RBBB
Long or short QT syndrome
Brugada-like early repolarization
Echocardiographic Changes in Athletes
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Sudden Cardiac Deaths in Athletes, Including Commotio Cordis