Sudden Cardiac Death
Andrew E. Darby, MD
John P. DiMarco, MD, PhD
 ESSENTIALS OF DIAGNOSIS
ESSENTIALS OF DIAGNOSIS
![]() Unexpected death occurring within an hour of onset of symptoms.
Unexpected death occurring within an hour of onset of symptoms.
![]() Primary electrical mechanisms include ventricular fibrillation, ventricular tachycardia, asystole, and pulseless electrical activity.
Primary electrical mechanisms include ventricular fibrillation, ventricular tachycardia, asystole, and pulseless electrical activity.
 General Considerations
General Considerations
Each year in the United States, more than 350,000 individuals die suddenly of some form of cardiovascular disease. Because of the many advances made during the past 30 years in clinicians’ ability to identify and modify the risk factors associated with sudden death, to resuscitate victims of cardiac arrest, and to prescribe specific antiarrhythmic therapy to prevent recurrences, age-adjusted sudden death mortality rates have declined dramatically. However, the number of elderly individuals in the population has increased, and sudden cardiac arrest remains an important problem.
For general clinical purposes, the term “sudden cardiac death” is usually reserved for those deaths in which the patient had stable cardiac function until the terminal event, with death occurring within a short time (often defined as less than 1 hour) of the onset of symptoms. Some experts prefer the term “instantaneous death,” namely, death with immediate collapse without preceding symptoms. Instantaneous death is usually assumed to be due to a primary arrhythmia, but other catastrophic events, such as a massive pulmonary embolism, the rupture of an aortic aneurysm, or a stroke, can also cause instantaneous death. It is also important to note that not all arrhythmic deaths are sudden. For example, a patient who is resuscitated from a cardiac arrest may die days or weeks later from complications of the arrest. This death would be due to an arrhythmia but would not meet the standard time-based definition for instantaneous or sudden death.
Effective evaluation and treatment of patients at risk for cardiac arrest and sudden death require an understanding of the responsible pathophysiologic mechanisms, the strategies proposed for primary prevention, the techniques and results of resuscitation, and the treatment modalities for secondary prevention in survivors of an initial episode.
Roger VL, et al. Heart disease and stroke statistics – 2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–220. [PMID: 22179539]
 Pathophysiology & Etiology
Pathophysiology & Etiology
A number of different electrophysiologic mechanisms may be responsible for sudden cardiac death. When ambulatory electrocardiographic (ECG) recordings from the time of an out-of-hospital cardiac arrest are examined, ventricular fibrillation and rapid ventricular tachycardia are the most commonly documented initial arrhythmias. Bradyarrhythmias, including atrioventricular block, asystole, or electromechanical dissociation, are also observed. The prevalence of these latter arrhythmias is higher in the setting of progressive and advanced underlying heart disease; in the elderly, and in patients whose sudden death is precipitated by an acute catastrophe, such as a pulmonary embolism, an acute myocardial infarction, rupture of a major vessel, or a major neurologic insult. Extrapolation of the mortality rate from data recorded by the Resuscitation Outcomes Consortium suggests that 382,800 people experience out-of-hospital cardiac arrests in the United States each year. Among out-of-hospital cardiac arrests treated by emergency medical personnel, only 23% have an initial rhythm of ventricular tachycardia or ventricular fibrillation or are shockable by an automated external defibrillator. Interestingly, the incidence of cardiac arrest with an initial rhythm of ventricular fibrillation has decreased over time, although the incidence of cardiac arrest with any rhythm has not decreased. The focus of this chapter will be principally those sudden deaths for which an arrhythmia was the primary cause.
A. Coronary Artery Disease
Although sudden death occurs in all forms of heart disease, in the United States and Europe, coronary artery disease is the most common cardiac diagnosis seen in sudden death victims (Table 15–1). Several mechanisms can produce potentially fatal arrhythmias among patients with coronary artery disease, and it is often difficult to define the precise factors that gave rise to a given episode. At one extreme is the patient with a previously normal ventricle who has an acute occlusion of a major epicardial coronary artery in whom ventricular fibrillation then develops during the first minutes of an acute infarction. This patient represents an example of pure ischemic injury without associated prior scar. At the other end of the spectrum is the patient with a history of a single-vessel occlusion and an old myocardial infarction, in whom postinfarction scarring has provided the anatomic substrate for a rapid reentrant ventricular tachycardia that results in hemodynamic collapse and sudden death. Acute ischemia need not be involved as a trigger in the latter situation. In coronary artery disease, the individuals at highest risk for sudden death have both multivessel disease and myocardial scarring from one or more prior infarctions. Even in such individuals, sudden cardiac arrest may be the first clinical manifestation of the disease. As treatment of acute myocardial infarction has become more aggressive during the past 20 years, the nature of the typical scar that results from a myocardial infarction has also changed. Dense scar tissue with aneurysm formation, the classic substrate associated with uniform morphology ventricular tachycardia, is now seen less often. After early pharmacologic or mechanical reperfusion, the current standards of therapy, the infarct zone shows mostly patchy fibrosis, and in such areas, disorganized arrhythmias predominate. In patients with this complex substrate, sudden death is thought to result from a complex interaction between some triggering event, such as ischemia, autonomic nervous system dysfunction, electrolyte imbalance, or drug toxicity, and the unstable electrophysiologic milieu created by prior infarction.
Table 15–1. Cardiac Conditions Associated with Sudden Death

Autopsy and clinical studies have highlighted this complexity. Coronary artery thrombi or plaque rupture may be detected in up to 50% of sudden death victims, but new Q-wave myocardial infarctions will develop in only about 25% of patients resuscitated from an out-of-hospital cardiac arrest. Angiographic studies in cardiac arrest survivors have shown that a high proportion of persons have long, diffusely irregular, and ulcerated coronary lesions similar to those seen in patients with acute coronary syndromes. It has also been demonstrated that therapy directed at ischemia reduces the incidence of sudden death. Aggressive surgical revascularization has been shown to decrease late sudden death mortality. In the Coronary Artery Bypass Graft (CABG-Patch) trial, no survival benefit over control was seen in patients who received an implantable cardioverterdefibrillator (ICD) at the time of their revascularization surgery. Based on this confusing overall picture, it is prudent in any individual with coronary disease to consider ischemia as an important, potentially reversible risk factor for sudden death, even in the absence of clinical angina. In previously asymptomatic individuals, coronary artery disease may still be the cause of sudden death. Significant coronary artery disease may be asymptomatic or unrecognized, and the general population contains a large number of such individuals. Up to 50% of all sudden cardiac deaths due to coronary artery disease may occur in individuals not previously known to have the condition.
Other diseases of the coronary arteries are rare causes of sudden death. An anomalous origin of a coronary artery may give rise to either myocardial scarring with late ventricular tachycardia or to arrhythmias mediated by acute intermittent ischemia. Similar mechanisms affecting patients with coronary artery spasm, embolism, trauma, dissections, or arteritis may cause sudden death.
B. Hypertrophic Cardiomyopathy
Hypertrophic cardiomyopathy is the most common genetic cardiovascular disorder with an estimated prevalence of 1 in 500. In hypertrophic cardiomyopathy, sudden death tends to occur in young adults who often have had no prior cardiac symptoms. There appears to be an excess of events during vigorous exercise, and hypertrophic cardiomyopathy is the leading cause of sudden death among competitive athletes in the United States. Teenagers or young adults in some kindreds with familial hypertrophic cardiomyopathy have a higher incidence of sudden death than do older members. In other families, sudden death in young adults is uncommon but may occur after heart failure has developed.
Several clinical risk factors for sudden death in patients with hypertrophic cardiomyopathy have been determined. These include a family history of sudden death; recurrent, unexplained syncope; nonsustained ventricular tachycardia during ambulatory monitoring; hypotension during exercise; and severe (> 30 mm) left ventricular hypertrophy. The presence of myocardial scarring on a magnetic resonance scan is a new risk factor that has recently been identified. Hypertrophic cardiomyopathy is a genetic disease primarily affecting the cardiac sarcomere with 60–70% of cases accounted for by sarcomere mutations. Genetic studies of patients with hypertrophic cardiomyopathy have revealed more than 900 mutations in 13 different genes. Some mutations (eg, those in troponin T) may be associated with a high risk of sudden death even in the absence of marked left ventricular hypertrophy. Polymorphic ventricular tachycardia or ventricular fibrillation, rather than monomorphic ventricular tachycardia with a scar-related intramyocardial circuit, is thought to be the initial arrhythmia at the time of cardiac arrest in patients with hypertrophic cardiomyopathy. Due to the severe hypertrophy and conduction system disease seen in patients with hypertrophic cardiomyopathy, sustained ventricular tachycardia due to reentry in the His-Purkinje system may occur and result in hemodynamic collapse with sudden death. Patients with hypertrophic cardiomyopathy are also at risk for sudden death due to atrioventricular block and supraventricular arrhythmias because any change in rhythm that produces significant ischemia in the hypertrophied ventricular wall may degenerate to a fatal arrhythmia.
C. Nonischemic Dilated Cardiomyopathy
Nonischemic dilated cardiomyopathy is the primary cardiac diagnosis in about 10% of patients who have been resuscitated after cardiac arrest. Sudden death accounts for about half of all deaths in patients with this diagnosis. In contrast to the situation in some forms of hypertrophic cardiomyopathy, sudden death tends to occur relatively late in the course of dilated cardiomyopathy, after hemodynamic symptoms have been present for some time. A variety of arrhythmias have been implicated in patients with this condition; both monomorphic and polymorphic ventricular tachycardias are seen in patients with nonischemic dilated cardiomyopathies. Intraventricular conduction delays may lead to ventricular tachycardia caused by macroreentry in the His-Purkinje system. In patients with this arrhythmia, catheter ablation of one of the bundle branches may be curative. In patients with cardiomyopathies and very advanced heart failure, brady-arrhythmias, rather than tachyarrhythmias, are the initial recorded rhythm in up to 50% of cardiac arrests. Some forms of familial dilated cardiomyopathy (eg, lamin A/C mutation carriers) are associated with markedly increased risks for sudden death, and such patients will often require intervention outside of standard guidelines.
D. Other Cardiac Diseases
In valvular heart disease, sudden death can occur in several ways. Sudden death is usually related to exertion in young adults with congenital aortic stenoses. In other forms of valvular heart disease, sudden death is usually a late occurrence seen in patients with advanced heart failure and ventricular hypertrophy. Although symptomatic atrial and ventricular arrhythmias are common in patients with mitral valve prolapse, truly life-threatening arrhythmias are rare, except in the presence of some complicating condition, such as a LQTS, electrolyte imbalance, or drug toxicity. In pulmonary hypertension, sudden death may occur from hemodynamic causes, bradyarrhythmias, or tachyarrhythmias.
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is a regional myopathy with primarily right ventricular involvement. When genetic studies have been performed, mutations in one of the desmosomal proteins are often found. These patients usually have left bundle branch block morphology ventricular tachycardias. Symptoms and signs of right ventricular dysfunction may or may not be present in patients with ARVC and ventricular tachycardia, and the clinical course is highly variable. It has been suggested that ARVC with extensive disease (including left ventricular involvement), one or more affected family members with sudden death, or undiagnosed syncope (when ventricular tachycardia or ventricular fibrillation has not been excluded as the cause of syncope) may indicate an increased risk of sudden death and prompt consideration for a primary prevention ICD.
In most forms of congenital heart disease, sudden arrhythmic death in the absence of severe heart failure, ventricular hypertrophy, or hypoxemia is uncommon. However, late ventricular tachycardia that arises from the right ventriculotomy scar or the septal repair may develop in some patients who have undergone a successful surgical repair of Fallot tetralogy.
E. Inherited Arrhythmia Syndromes
The congenital long QT syndrome (LQTS) is a family of disorders characterized by prolongation of cardiac repolarization with a prolonged QT interval on the scalar ECG and a tendency to develop polymorphic ventricular tachycardia that may degenerate to ventricular fibrillation. It occurs with an estimated prevalence of 1 in 3000 to 5000 in the general population. The most common types of the LQTS are caused by mutations in genes that encode ion channel proteins. The resultant ion channel dysfunction causes a prolonged repolarization phase of the ventricular action potential. This promotes polymorphic ventricular tachycardia triggered by oscillations in the action potential called early after-depolarizations. Electrolyte imbalance, bradycardia or pauses, sudden sympathetic stimulation, and drug effects all may further prolong repolarization in individuals with these mutations and trigger acute episodes. Factors significantly affecting outcome include QTc duration (ie, increased risk of ventricular arrhythmias with QTc > 500 ms); age-gender interactions (increased event rate in males during childhood and females after onset of adolescence); LQTS genotype (LQT3 responds less well to β-blocker therapy); and syncope despite treatment with a β-blocker. Interestingly, family history of sudden death does not seem to be a marker of life-threatening cardiac events in LQTS patients. It is important to recognize patients with LQTS because standard antiarrhythmic drugs may worsen their condition and other QT-prolonging agents must be avoided.
A short QT syndrome caused by a gain in function mutation in a repolarizing potassium current that is associated with sudden death has also been described. The syndrome is characterized by short QT intervals (< 350 ms) and high incidence of syncope, sudden death, and atrial fibrillation.
The Brugada syndrome is another familial condition associated with sudden death. These individuals have an incomplete or complete right bundle branch block on their ECG with ST segment elevation in V1 and V2. These patients will manifest spontaneous episodes of polymorphic ventricular tachycardia and ventricular fibrillation, often during sleep. Some patients with Brugada syndrome have a mutation in the sodium channel gene (SCN5A) with a decrease in the inward sodium current during the plateau phase of the action potential. The unusual ECG manifestations are believed to be due to more pronounced ion channel dysfunction in the right ventricular epicardium.
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is a rare syndrome in which bursts of rapid ventricular tachycardia occur during sympathetic stimulation or exercise. This syndrome is genetically heterogenous, with mutations in the genes encoding the cardiac ryanodine receptor type II and calsequestrin. These genetic mutations result in deranged calcium homeostasis with increased intracellular calcium, thereby predisposing to an increased risk of ventricular arrhythmias. β-Blockers are indicated for symptomatic patients. An ICD may be indicated for CPVT patients with syncope or sustained ventricular tachycardia despite treatment with β-blockers.
Advancements in genetic testing have facilitated the diagnosis and risk stratification of patients with inherited arrhythmia syndromes. Most of these diseases are caused by single genetic mutations that are heritable (often in an auto-somal dominant fashion). However, the genetic cause has been identified for only a portion of these life-threatening disorders (up to 75% of LQTS cases; 65% of hypertrophic cardiomyopathy; 50% of ARVC and CPVT; and 25 to 30% of dilated cardiomyopathy and Brugada syndrome). Genome-wide association studies (GWAS) analyze the entire human genome in large cohorts seeking to establish a correlation between common genetic mutations and disease. Specifically, GWAS assess the impact of single nucleotide polymorphisms (SNPs; genetic mutations that are common [> 1%] in the general population) on the susceptibility to arrhythmias. GWAS have identified mutations that affect the QT interval and may modulate the risk of sudden cardiac death in the LQTS. Further research may identify mutations that influence the risk of sudden death in other cardiac conditions as well as the susceptibility to drug-induced QT prolongation and torsade de pointes.
Barsheshet A, et al. Genetics of sudden cardiac death. Curr Cardiol Rep. 2011;13:364–76. [PMID: 21789574]
Cerrone M, et al. Genetics of sudden death: focus on inherited channelopathies. Eur Heart J. 2011;32:2109–20. [PMID: 21478491]
Milan DJ, et al. Genome-wide association studies in cardiac electrophysiology: recent discoveries and implications for clinical practice. Heart Rhythm. 2010;7:1141–8. [PMID: 20423731]
F. Drug-Induced Arrhythmias
Drug toxicity can also result in sudden death. A variety of medications can affect cardiac electrophysiology and lead to fatal arrhythmias. Even when prescribed for atrial fibrillation or supraventricular tachycardia, all antiarrhythmic drugs may be associated with a proarrhythmic response in the ventricle. Other cardiac and noncardiac drugs can also cause arrhythmias. The most common mechanism is IKr blockade. Multiple factors are often required for drug-induced proarrhythmia. Risk factors include electrolyte disturbances, age, female gender, genetic polymorphisms or mutations, left ventricular hypertrophy, and bradycardia.
Patients with severe electrolyte disturbances and abnormal dietary histories (eg, anorexia nervosa and liquid protein diets) are also susceptible to potentially fatal ventricular arrhythmias even in the absence of significant heart disease.
G. Other Arrhythmias
Several electrophysiologic abnormalities can produce sudden death without associated major structural heart disease. Supraventricular arrhythmias, if associated with very rapid ventricular rates, can cause hemodynamic collapse and degenerate to ventricular fibrillation. Atrial fibrillation with rapid conduction over an accessory pathway in a patient with Wolff-Parkinson-White syndrome is the supraventricular arrhythmia most frequently associated with sudden death, but other supraventricular arrhythmias have also occasionally been implicated. Although sudden death due to a ventricular preexcitation syndrome is rare, it may be the first clinical manifestation of the condition.
Bradyarrhythmias may also be associated with sudden death. In congenital complete heart block, the escape pacemaker may deteriorate over time, with ventricular arrhythmias appearing as the patient’s bradycardia becomes more and more inappropriate. Most previously healthy adults in whom a bradycardia develops as a result of sinus node dysfunction or heart block will have some functioning escape pacemaker that can, at least briefly, support vital organs. Therefore, sudden death is uncommon with these arrhythmias in the absence of severe ventricular dysfunction, another complicating disease, electrolyte imbalance, drug toxicity, or a prolonged delay in treatment of the bradycardia.
A recently recognized syndrome of sudden death in young individuals with structurally normal hearts is commotio cordis. Ventricular fibrillation develops after the patient receives a sharp blow to the chest, often while engaged in sports. Animal models have shown that a critically timed and placed chest impact during a vulnerable portion of the T wave can initiate ventricular fibrillation. It is assumed that a similar mechanism is responsible for the human syndrome.
Not all ventricular arrhythmias in patients with structurally normal hearts carry a risk for sudden death. Sudden death is very rare in individuals with structurally normal hearts who initially present with a stable monomorphic ventricular tachycardia. The two most common forms of sustained monomorphic ventricular tachycardia in patients with structurally normal hearts arise either from the right ventricular outflow tract with a left bundle branch block pattern and an inferior axis or from the inferior septal region with a right bundle branch block and left axis pattern. Both of these forms of ventricular tachycardia are usually hemodynamically well tolerated and rarely result in sudden cardiac death.
 Management of Cardiac Arrest: Initial Resuscitation
Management of Cardiac Arrest: Initial Resuscitation
The introduction of transthoracic defibrillation 50 years ago sparked the development of community-based programs to resuscitate persons who suffered cardiac arrest out of the hospital. The most successful systems involve an educated lay public that can provide basic cardiopulmonary resuscitation (CPR), judicious community placement of automatic external defibrillators (AEDs) for nonprofessional use, and trained responders who can provide advanced life support in the field. The most important factor influencing survival is the time from cardiac arrest to restoration of an organized cardiac rhythm. If an effective rhythm is not restored within 4–8 minutes, survival with well-preserved neurologic function becomes unlikely. Bystander CPR can extend this window for survival by a few minutes.
Because early defibrillation is the key to survival, community programs to speed defibrillation have been widely introduced but with modest results. Initial efforts involved emergency medical technicians trained in both basic and advanced cardiac life support who responded to the emergency call. The success of these programs was limited by the ability of these trained responders to reach the patient within the first critical minutes after the arrest.
Public access to AEDs has been shown to improve survival for out-of-hospital cardiac arrest patients. When an AED is connected to an unconscious individual by electrode pads placed on the chest, a microprocessor within the device analyzes the patient’s rhythm. Ventricular fibrillation and rapid ventricular tachycardia are accurately identifiable as “shockable” rhythms, and the AED instructs the rescuer to push a button to deliver a shock. AEDs designed for home use by minimally trained lay family members are now commercially available, and a wearable vest AED that does not require a rescuer for activation has recently been introduced. The greatest success with AED usage has been in public places like casinos, sports venues, and transportation centers where arrest victims are likely to be observed quickly and security personnel trained in AED usage are present. Basic CPR techniques have also evolved, with the current emphasis placed on maintenance of effective chest compression.
A discussion of the techniques for basic and advanced cardiac life support is beyond the scope of this chapter. For patients with ventricular tachycardia or fibrillation, early cardioversion or defibrillation is the key to survival. For patients with asystole or pulseless electrical activity, the prospects for survival remain poor unless some reversible cause can be identified and immediately corrected.
 Management of Cardiac Arrest Survivors: In-Hospital Phase
Management of Cardiac Arrest Survivors: In-Hospital Phase
Even in communities that have effective programs for pre-hospital cardiac care, only a fraction of cardiac arrest patients will survive to hospital admission. The immediate goal for rescuers in the field is restoration of spontaneous circulation. If that can be achieved, preservation of the brain, heart, and other vital organs must be considered. Potential complications of the resuscitation must be identified and treatment instituted. The probable cause, including reversible precipitating events, the nature and severity of any underlying heart disease, and the arrhythmia probably responsible for the episode, should be determined. Finally, therapy can be selected and its potential for success evaluated.
A. Complications of Resuscitation
Only a fraction of cardiac arrest survivors who receive early defibrillation will be alert and oriented with full recovery of function at the time of hospital admission. Most patients will have pulmonary, cardiac, or neurologic complications resulting from the period of arrest or the resuscitation itself. Pulmonary complications are usually due either to aspiration of gastric contents or to mechanical injury to the thoracic cage during closed-chest compressions. The chest wall should be carefully inspected, palpated, and stabilized, if necessary. In extreme cases, bony thoracic fractures may result in a flail chest, or hepatic or splenic lacerations may occur. Chest radiography may be helpful in detecting aspiration, but repeated examinations may be necessary to document the delayed appearance of infiltrates. If a central line has been placed, the chest radiograph is also useful to confirm catheter position and to exclude a pneumothorax. Mechanical ventilation is often required in the early period after admission to allow adequate oxygenation and pulmonary cleansing; this may require the use of muscle relaxants and sedation. Over oxygenation should be avoided.
Early revascularization should be attempted in all cardiac arrest survivors who present with a new ST segment elevation myocardial infarction and should be considered whenever acute ischemia was a likely contributor to the arrest. Some cardiac arrest centers have urged that all cardiac arrest survivors undergo coronary angiography immediately after resuscitation, but this may not be possible in all hospitals. If an acute total coronary occlusion is identified, revascularization is indicated.
Cardiac arrest even without a new coronary occlusion produces a period of global cardiac ischemia, frequently resulting in a period of cardiac stunning, defined as a reversible depression in cardiac systolic function. Inotropic or even mechanical (eg, intra-aortic balloon counterpulsation) support may be necessary to maintain vital organ perfusion during the early phase after resuscitation. Any acute assessment of ventricular function may overestimate the amount of permanent dysfunction, and a low ejection fraction measured in the first several days after arrest may not be an accurate gauge of eventual cardiac function. Arrhythmias are frequently seen during the period immediately after resuscitation. They may be similar to those that originally produced the arrest, or they may be new rhythm disturbances caused by poor hemodynamic function and multiorgan failure. No single therapy will be predictably effective against these arrhythmias, and antiarrhythmic agents, β-adrenergic blockers, positive inotropic agents, and other measures to improve hemodynamic function must be tried. Recent studies using intravenous amiodarone prior to hospital admission have demonstrated improvements in rates of return of spontaneous circulation and survival-to-hospital admission but no clear benefit in survival-to-hospital discharge.
If spontaneous circulation can be restored and the patient is admitted to the hospital, neurologic damage is the major cause of death and long-term disability. Neurologic damage occurs quickly during a cardiac arrest. Unless defibrillation with restoration of spontaneous circulation was almost immediate, patients will be unconscious when admitted to the hospital, and an accurate evaluation of the potential for functional recovery is often difficult in this early stage. Brainstem reflexes may be preserved, but their presence does not necessarily predict a favorable outcome. Generalized or focal seizure activity, decerebrate or decorticate posturing, and involuntary respiratory efforts may make mechanical ventilation difficult. Neuromuscular blocking agents, anticonvulsants, and sedation are often required, further hampering any ability to make an accurate neurologic assessment. Studies have demonstrated that mild therapeutic hypothermia (32–34°C for 24 hours) significantly improves neurologic recovery in unconscious resuscitated cardiac arrest patients. Advanced life support protocols now call for therapeutic cooling to be started in the field or immediately upon hospital arrival (Figure 15–1). In patients who received therapeutic hypothermia, the prognosis is good if they regain consciousness within 72 hours of arrest. Many will recover completely with minimal or no long-term neurologic impairment. Therapeutic hypothermia does not rule out early revascularization, and both strategies should be employed. If coma persists longer than 72 hours, only a minority of patients survive. Those who do will often have persistent severe motor and cognitive deficits. Somatosensory evoked potential testing, measurement of brain-specific enolases, and electroencephalogram data may help to determine prognosis. Decisions about prolonged artificial support of these latter patients are often difficult and require that a variety of medical, ethical, and social factors be taken into consideration.
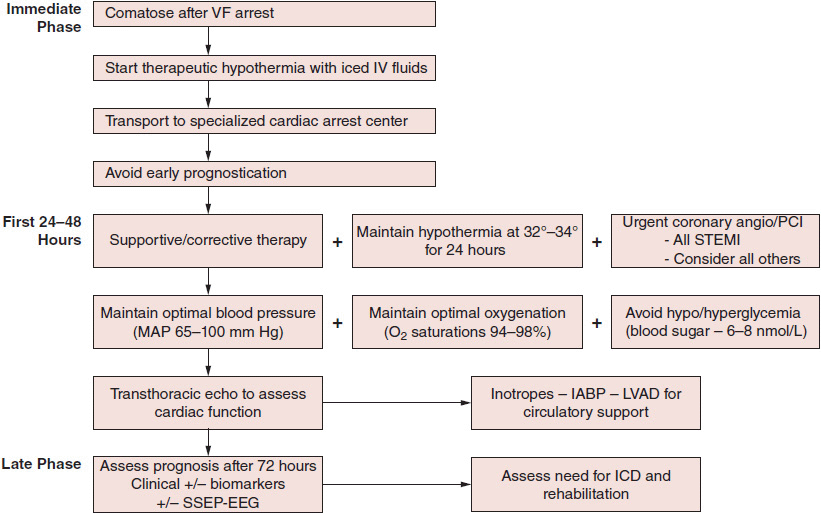
![]() Figure 15–1. Proposed management algorithm for out-of-hospital cardiac arrest victims. IABP, intra-aortic balloon pump; ICD, implantable cardioverter-defibrillator; IV, intravenous; LVAD, left ventricular assist device; MAP, mean arterial pressure; PCI, percutaneous coronary intervention; SSEP-EEG, somatosensory evoked potential–electroencephalography; STEMI, ST elevation myocardial infarction; VF, ventricular fibrillation.
Figure 15–1. Proposed management algorithm for out-of-hospital cardiac arrest victims. IABP, intra-aortic balloon pump; ICD, implantable cardioverter-defibrillator; IV, intravenous; LVAD, left ventricular assist device; MAP, mean arterial pressure; PCI, percutaneous coronary intervention; SSEP-EEG, somatosensory evoked potential–electroencephalography; STEMI, ST elevation myocardial infarction; VF, ventricular fibrillation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


