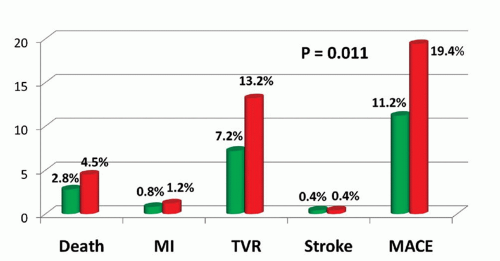
HR: 0.40; 95% CI: 0.13-1.27; p = 0.11); and MACE (6.8% vs. 9.4%; HR: 0.72; 95% CI: 0.48-1.08; p = 0.12) (1).
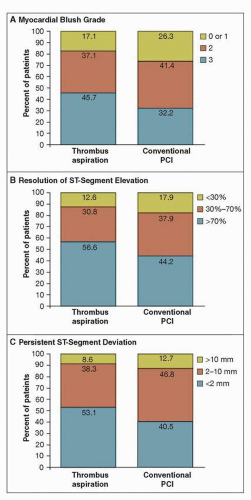 FIGURE 19-1 Myocardial reperfusion data on angiography and electrocardiography, according to treatment group.The percentages of patients are shown according to myocardial blush grade on the angiogram (Panel A) and the degree of resolution of ST-segment elevation (Panel B) and persistent ST-segment deviation (Panel C) on the electrocardiogram. PCI denotes percutaneous coronary intervention. Thrombectomy significantly improved markers of myocardial reperfusion (blush and ECG changes), with a strong 30-day trend toward a reduction in mortality. Note that 1-year mortality was significantly reduced (from 7.6% to 4.7%, p = 0.042). (From: Svilaas T, et al. N Engl J Med. 2008;358(6):557-567, with permission.) |
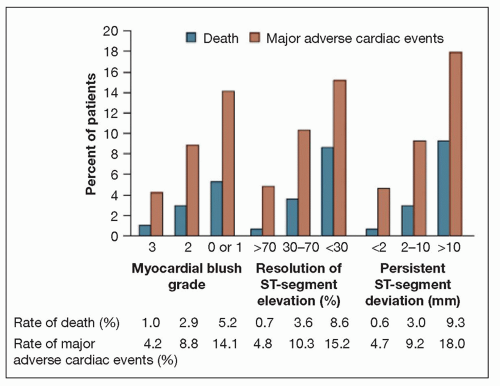 FIGURE 19-2 Rates of death and major adverse cardiac events, according to myocardial blush grade and ST-segment variables. Rates are shown for the 968 patients. p = 0.003 for the association between myocardial blush grade and death. p < 0.001 for all other associations: between myocardial blush grade and major adverse cardiac events, between ST-segment resolution and death, between ST-segment resolution and major adverse cardiac events, between persistent ST-segment deviation and death, and between persistent ST-segment deviation and major adverse cardiac events. These data support the use of surrogate markers of reperfusion. (From: Svilaas T, et al. N Engl J Med. 2008;358(6):557-567, with permission.) |
two groups, despite the fact that embolic debris was removed from 73% of patients randomized to distal protection. Similar clinical outcomes were seen in the ASPARAGUS trial (6) and others investigating embolic protection devices (Table 19-1). Thus, while embolic protection may be useful in situations of high thrombus burden, where there is significant concern for distal embolization, the routine use of embolic protection is not recommended in primary PCI for STEMI patients.
TABLE 19-1 Randomized Trials of Distal Protection Devices in Primary PCI | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree